Alkaline anion exchange membrane based on chemical cross-linking and preparation method thereof
A basic anion, chemical cross-linking technology, applied in the field of basic anion exchange membrane and preparation, can solve the problem of insufficient durability of anion exchange membrane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1) Weigh 4g of SEBS and dissolve it in 150mL of dichloromethane, stir it mechanically, after it is completely dissolved, add 3mL of N-methyl-4-piperidone, the solution becomes clear and light yellow.
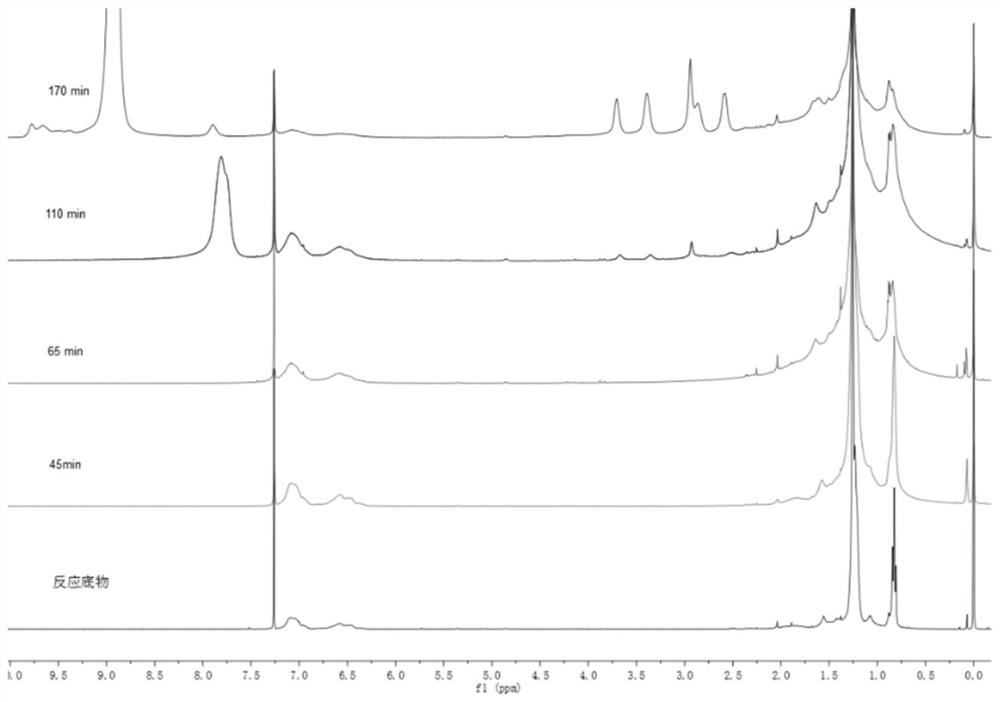
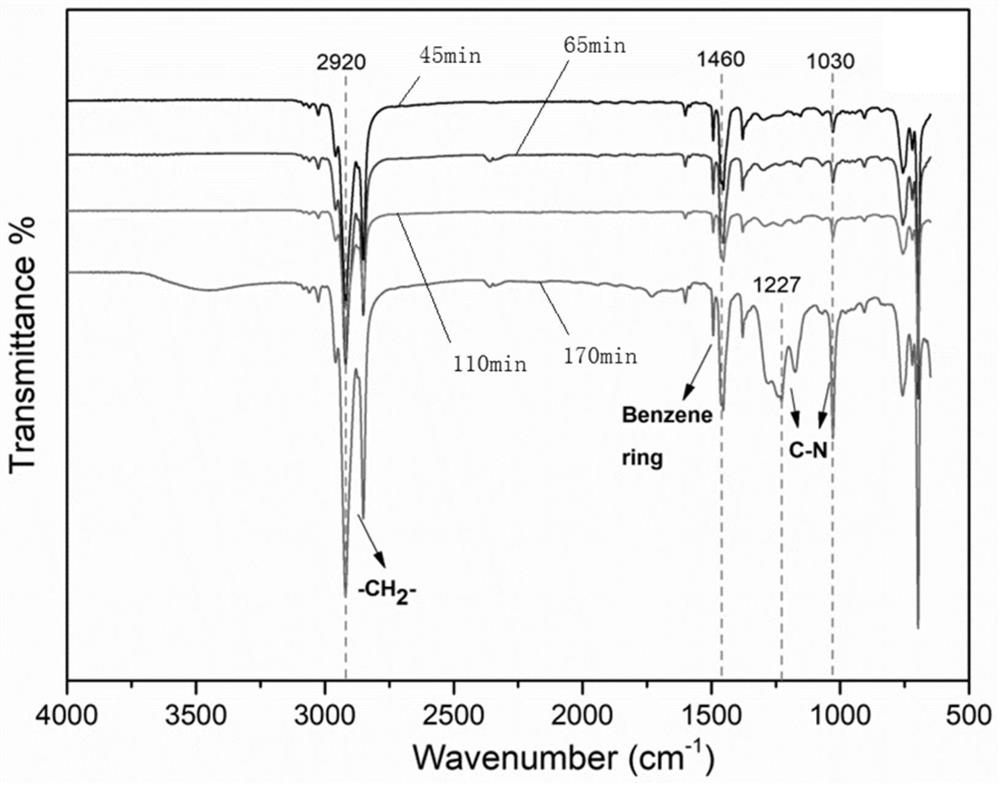
[0075] 2) Start the ice-water bath reaction. When the temperature drops to about 3-9°C, slowly add 15mL TFSA (trifluoromethanesulfonic acid) and 3mL TFA (trifluoroacetic acid) for 45 minutes. The liquid becomes viscous and the reaction is stopped.
[0076] 3) Pour the light brown liquid from the previous step into deionized water to quench the reaction, stir it mechanically at high speed, crush the viscous polymer, then wash it with deionized water for 4 to 8 times to neutrality, and dry it in vacuum to obtain a chemical cross-linked product. Linked polymer solids.
[0077] 4) Weigh 300 mg of the polymer solid in the previous step, dissolve it in 100 mL of chloroform, and after it is completely dissolved, keep the reaction system in a dark environment, and add 270 mg of K...
Embodiment 2
[0092] 1) Weigh 4g of SEBS and dissolve it in 150mL of dichloromethane, stir it mechanically, after it is completely dissolved, add 3mL of N-methyl-4-piperidone, the solution becomes clear and light yellow.
[0093] 2) Start the reaction in an ice-water bath, and when the temperature drops to about 3-9°C, slowly add 15mL TFSA and 3mL TFA, and react for 65min, the liquid becomes viscous, and the reaction is stopped.
[0094] 3) Pour the light brown liquid from the previous step into deionized water to quench the reaction, stir it mechanically at high speed, crush the viscous polymer, then wash it with deionized water for 4 to 8 times to neutrality, and dry it in vacuum to obtain a chemical cross-linked product. Linked polymer solids.
[0095] 4) Weigh 300 mg of the polymer solid in the previous step, dissolve it in 200 mL of chloroform, and after it is completely dissolved, keep the reaction system in a dark environment, and add 270 mg of K 2 CO 3 , 0.18mL methyl iodide, reac...
Embodiment 3
[0101] 1) Weigh 4g of SEBS and dissolve it in 150mL of dichloromethane, stir it mechanically, after it is completely dissolved, add 3mL of N-methyl-4-piperidone, the solution becomes clear and light yellow.
[0102] 2) Start the reaction in an ice-water bath, and when the temperature drops to about 3-9°C, slowly add 15mL TFSA and 3mL TFA, and react for 110min, the liquid becomes viscous, and the reaction is stopped.
[0103] 3) Pour the light brown liquid from the previous step into deionized water to quench the reaction, stir it mechanically at high speed, crush the viscous polymer, then wash it with deionized water for 4 to 8 times to neutrality, and dry it in vacuum to obtain a chemical cross-linked product. Linked polymer solids.
[0104] 4) Weigh 300 mg of the polymer solid in the previous step, dissolve it in 150 mL of chloroform, and after it is completely dissolved, keep the reaction system in a dark environment, and add 270 mg of K 2 CO 3 , 0.18mL methyl iodide, rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ion exchange capacity | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com