Quaternary ammonium salt polyphosphazene hydrogel wound dressing with active and passive dual antibacterial mechanisms and preparation method
A wound dressing and hydrogel technology, applied in bandages, medical science and other directions, can solve the problems of strong bacterial resistance and biotoxicity, and achieve synergistic antibacterial efficiency, good swelling ability, improved load capacity and sustained release ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 56.1 mg of poly(dimethyl-aminoethyl-allyl ammonium bromide) phosphazene and 543.2 mg of 1-vinyl-2-pyrrolidone were dissolved in 1401.3 mg of deionized water to obtain a mixed solution. After being fully dissolved, argon gas was continuously passed into the mixed solution for 30 minutes to remove the oxygen in the mixed solution, and then 5.9 mg of 2-hydroxy-4-(2-hydroxyethoxy)-2-methyl was added to the mixed solution Propiophenone and fully dissolved to obtain a gel precursor solution.
[0041] The gel precursor solution was injected into the block mold (length = 1cm, width = 1cm, height = 1mm) and the columnar mold (inner diameter = 1cm), and then both the block mold and the columnar mold were placed under a 100W ultraviolet lamp for photoinitiation. Based polymerization reaction, the reaction lasted 30min. After the reaction, sheet-like and column-like organogels using deionized water as a solvent are obtained.
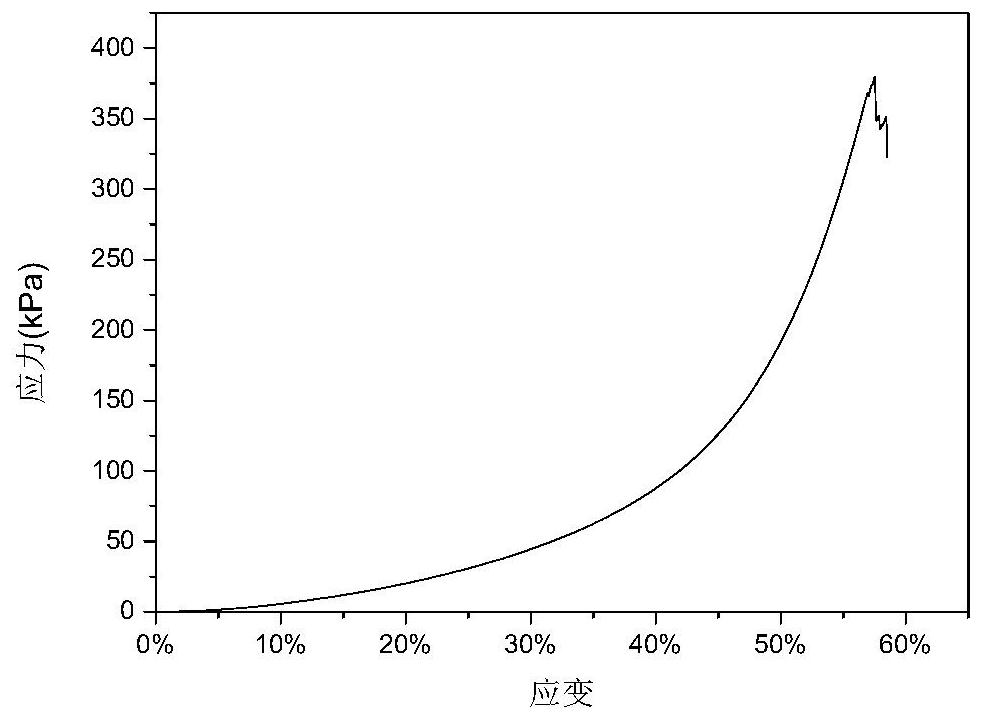
[0042] Take the sheet-shaped and columnar organogels ...
Embodiment 2
[0049] Poly(dimethyl-aminoethyl-allyl ammonium bromide) phosphazene 104.1 mg, 1-vinyl-2-pyrrolidone 495.5 mg, dissolved in 1400.5 mg deionized water to obtain a mixed solution. After being fully dissolved, argon gas was continuously passed into the mixed solution for 30 minutes to remove the oxygen in the mixed solution, and then 6.3 mg of 2-hydroxyl-4-(2-hydroxyethoxy)-2-methyl was added to the mixed solution Propiophenone and fully dissolved to obtain a gel precursor solution.
[0050] The gel precursor solution was injected into the block mold (length = 1cm, width = 1cm, height = 1mm) and the columnar mold (inner diameter = 1cm), and then both the block mold and the columnar mold were placed under a 100W ultraviolet lamp for photoinitiation. Based polymerization reaction, the reaction lasted 30min. After the reaction, sheet-like and column-like organogels using deionized water as a solvent are obtained.
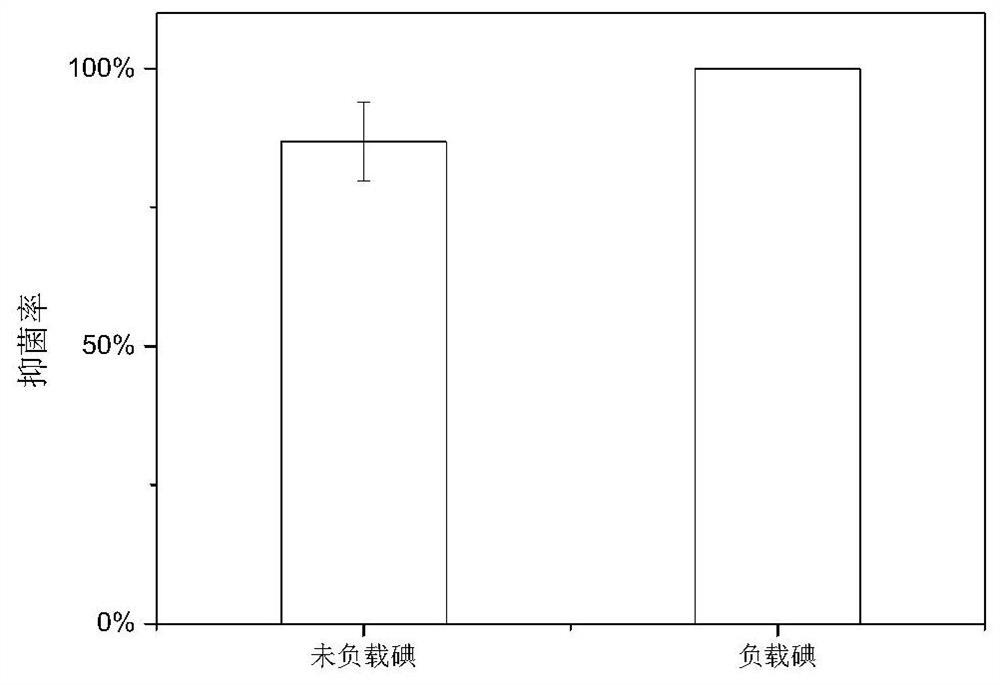
[0051] The resulting sheet and columnar hydrogels were placed in st...
Embodiment 3
[0057] 141.1 mg of poly(dimethyl-aminoethyl-allyl ammonium bromide) phosphazene, 460.5 mg of 1-vinyl-2-pyrrolidone were dissolved in 1402.5 mg of deionized water to obtain a mixed solution. After being fully dissolved, argon gas was continuously passed into the mixed solution for 30 minutes to remove the oxygen in the mixed solution, and then 6.0 mg of 2-hydroxyl-4-(2-hydroxyethoxy)-2-methyl was added to the mixed solution Propiophenone and fully dissolved to obtain a gel precursor solution.
[0058] The gel precursor solution was injected into the block mold (length = 1cm, width = 1cm, height = 1mm) and the columnar mold (inner diameter = 1cm), and then both the block mold and the columnar mold were placed under a 100W ultraviolet lamp for photoinitiation. Based polymerization reaction, the reaction lasted 30min. After the reaction, sheet-like and column-like organogels using deionized water as a solvent are obtained.
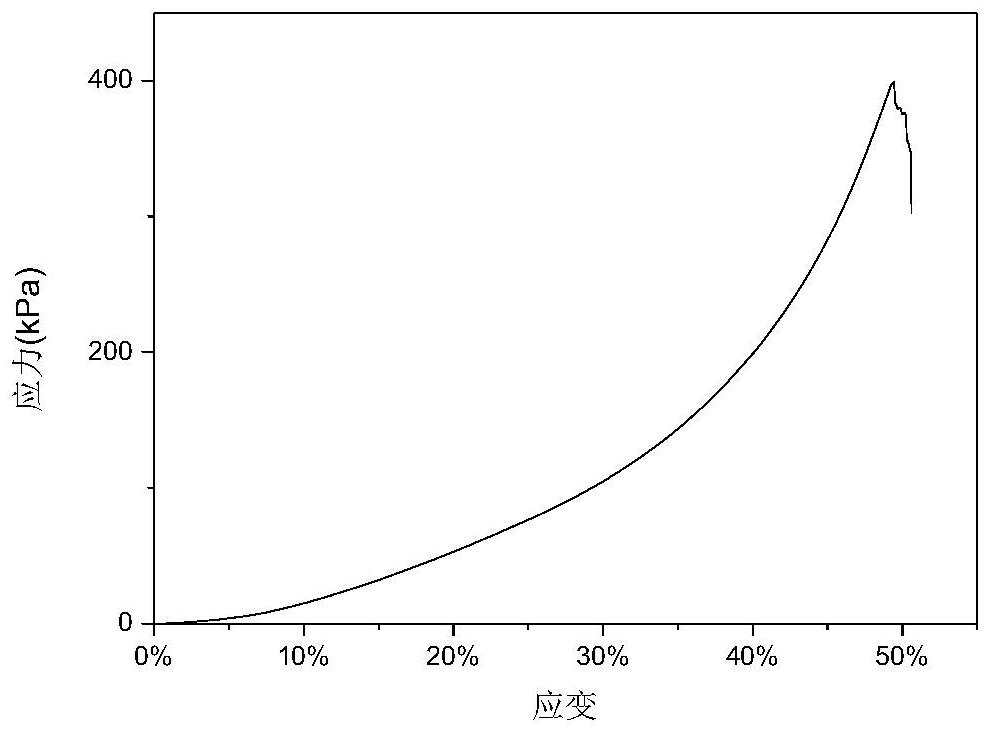
[0059] Take the sheet-shaped and columnar organogels o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com