Preparation method and application of Schwertmannite
A technology of Shi's minerals and a new method, which is applied in the field of water pollution control and can solve the problems of rare preparation costs and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The present embodiment provides the preparation method of Shi's mineral adsorbent, comprises the steps:
[0045] Step (1): Acid dissolves scrap iron to 9.2mol / L H 2 SO 4 Add 56g of scrap iron (gray cast iron HT300) to the solution. Put it in a water bath to keep a constant temperature of 80°C for reaction, and stir continuously to make it fully react. When the solution in the beaker no longer produces bubbles, the reaction is considered to be over. After the completely reacted solution is filtered with a Buchner funnel, it is sealed with a triangular flask save.
[0046] Step (2): Formation of Shi's minerals, Fe after dissolution of scrap iron 2+ The concentration is 210mmol, add 150mmol H to the solution in 6 times 2 o 2 , and reacted at room temperature for 12 hours. After the sample was collected by filtration and washing, the Schwartz mineral adsorbent was obtained, and the sample was dried and preserved.
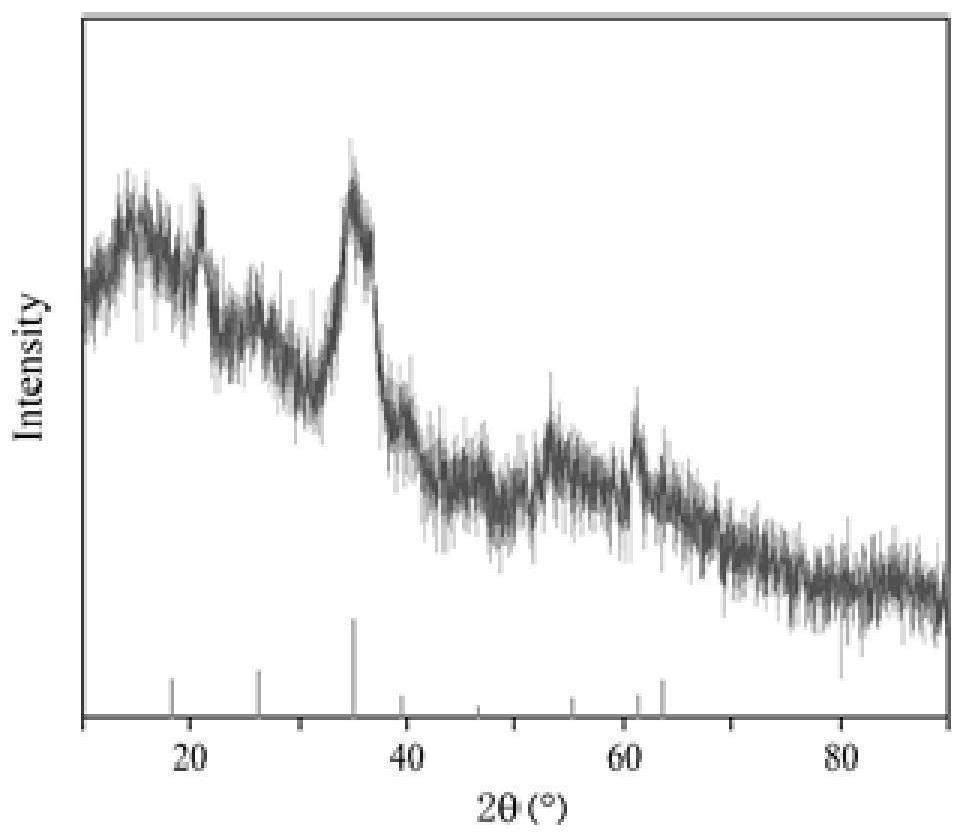
[0047] figure 1 It is the SEM image of the Shi's min...
Embodiment 2
[0050] This comparative example provides the preparation method of Shi's mineral adsorbent, comprises the steps:
[0051] Step (1): Acid dissolves scrap iron to 9.2mol / L H 2 SO 4 Add 56g of scrap iron (gray cast iron HT300) to the solution. Put it in a water bath to keep a constant temperature of 80°C for reaction, and stir continuously to make it fully react. When the solution in the beaker no longer produces bubbles, the reaction is considered to be over. After the completely reacted solution is filtered with a Buchner funnel, it is sealed with a triangular flask save.
[0052] Step (2): Formation of Shi's minerals, Fe after dissolution of scrap iron 2+ The concentration is 210mmol, add 150mmol H to the solution at one time 2 o 2 , reacted at room temperature for 12 hours. After the samples were collected by filtration and washing, the Schwartz mineral adsorbent was obtained, and the samples were preserved after drying.
[0053] The Shi Shi mineral that this comparati...
Embodiment 3
[0059] The present embodiment provides the preparation method of Shi's mineral adsorbent, comprises the steps:
[0060] Step (1): Acid dissolves scrap iron to 9.2mol / L H 2 SO 4Add 56g of scrap iron (gray cast iron HT300) to the solution. Put it in a water bath and keep it at a constant temperature of 70°C for reaction. Stir continuously to make it fully react. When the solution in the beaker no longer produces bubbles, it is considered that the reaction is over. save.
[0061] Step (2): Formation of Shi's minerals, Fe after dissolution of scrap iron 2+ The concentration is 210mmol, add 150mmolH to the solution 2 o 2 (adding in 6 times), react at room temperature for 4 hours. After the samples were collected by filtration and washing, the Schwartz mineral adsorbent was obtained, and the samples were preserved after drying.
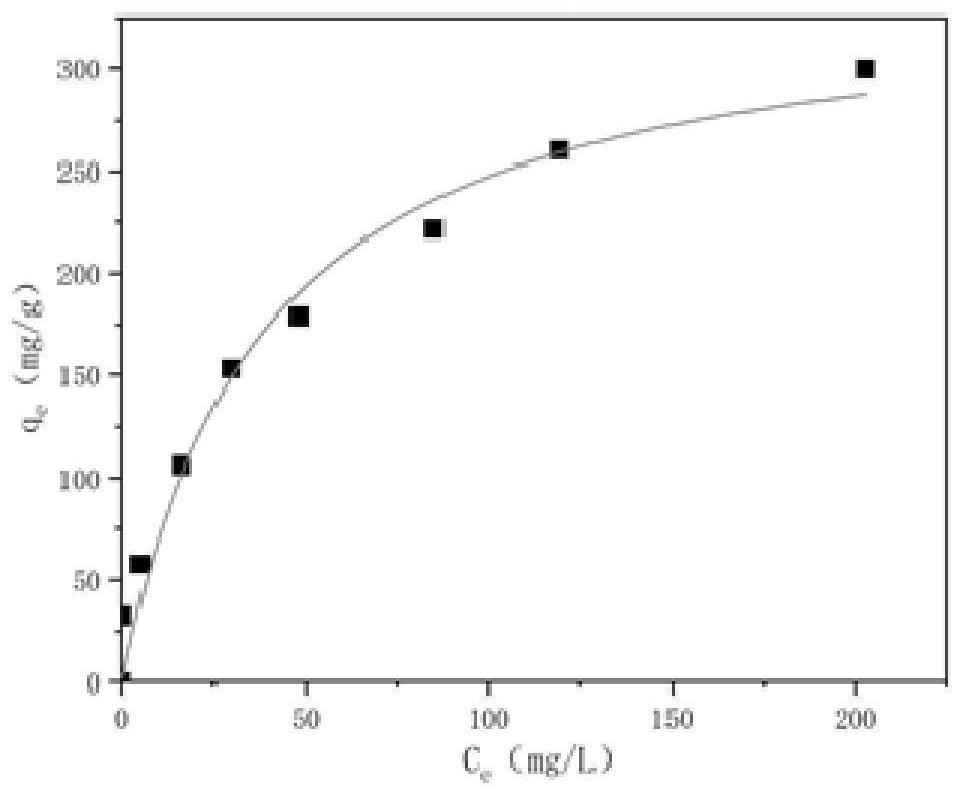
[0062] According to the results of the total iron precipitation rate measurement points, after scrap iron was prepared into Shi's minerals and react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com