Halogenated benzohydroxamic acid collecting agent and application thereof in mineral flotation
A technology of halogenated benzyl hydroxamic acid and o-fluorobenzoic hydroxamic acid, which is applied in the field of halogenated benzyl hydroxamic acid collectors, and can solve problems such as insufficient collection capacity of benzyl hydroxamic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This example provides a preparation method of o-fluorobenzoic hydroxamic acid, the steps are: mix 10.0 g of o-fluorobenzoic acid and 50 mL of methanol, then add 1.0 g of concentrated sulfuric acid catalyst, heat to 80 ° C, stir and reflux for 3 hours , obtain the methyl o-fluorobenzoate fraction by distillation under reduced pressure (80°C);
[0054] Dissolve 6.45 g of hydroxylamine hydrochloride in 30 mL of methanol solution, add sodium methoxide, and filter to remove the sodium chloride generated by the reaction to obtain hydroxylamine solution;
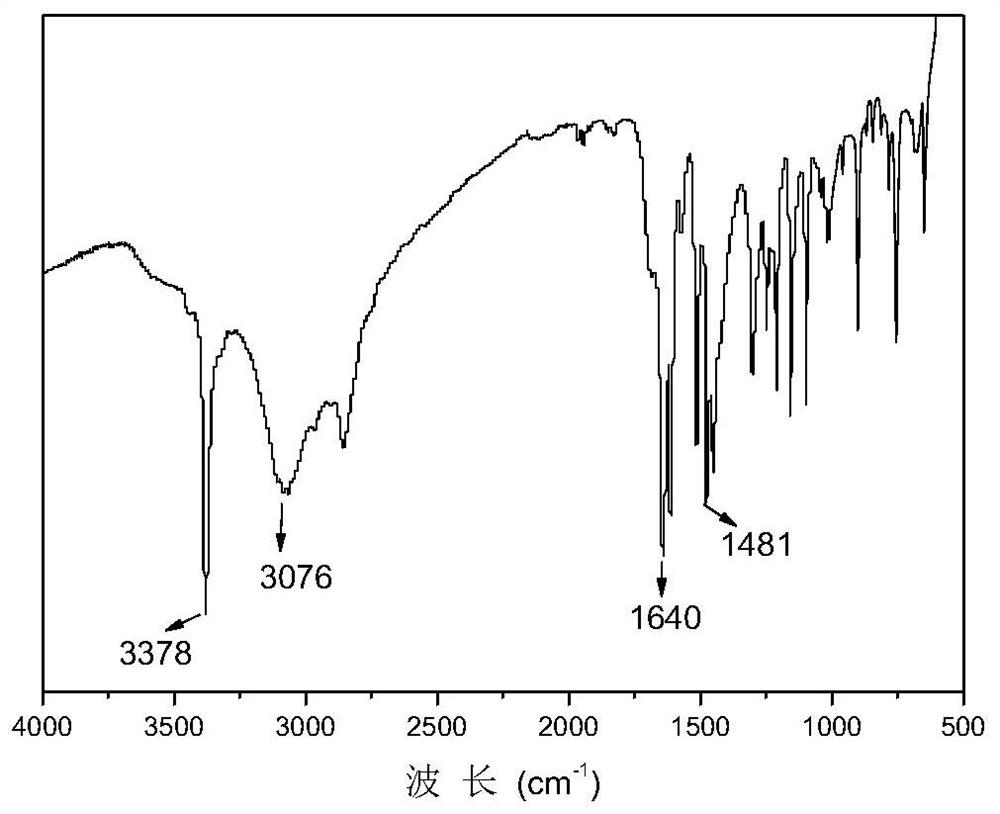
[0055] Mix the obtained methyl o-fluorobenzoate and hydroxylamine solution evenly, add sodium hydroxide methanol solution, react at 30°C for 5 hours, add concentrated sulfuric acid, and obtain o-fluorobenzoic acid hydroxamic acid, the structural formula is as follows, a total of 14.5g . The infrared spectrum of o-fluorobenzohydroxamic acid is shown in figure 1 .
[0056]
Embodiment 2
[0058] This embodiment provides a kind of preparation method of 3,4-difluorobenzoic acid, and the steps are: mix 10.0g 3,4-difluorobenzoic acid and 50mL ethanol, then add 1.0g benzenesulfonic acid catalyst, Heated to 90°C, stirred and refluxed for 5 hours, and obtained 3,4-difluorobenzoic acid ethyl ester fraction by vacuum distillation (100°C);
[0059] Dissolve 5.27 g of hydroxylamine hydrochloride in 30 mL of ethanol solution, add sodium ethoxide, filter to remove the sodium chloride generated by the reaction, and obtain hydroxylamine solution;
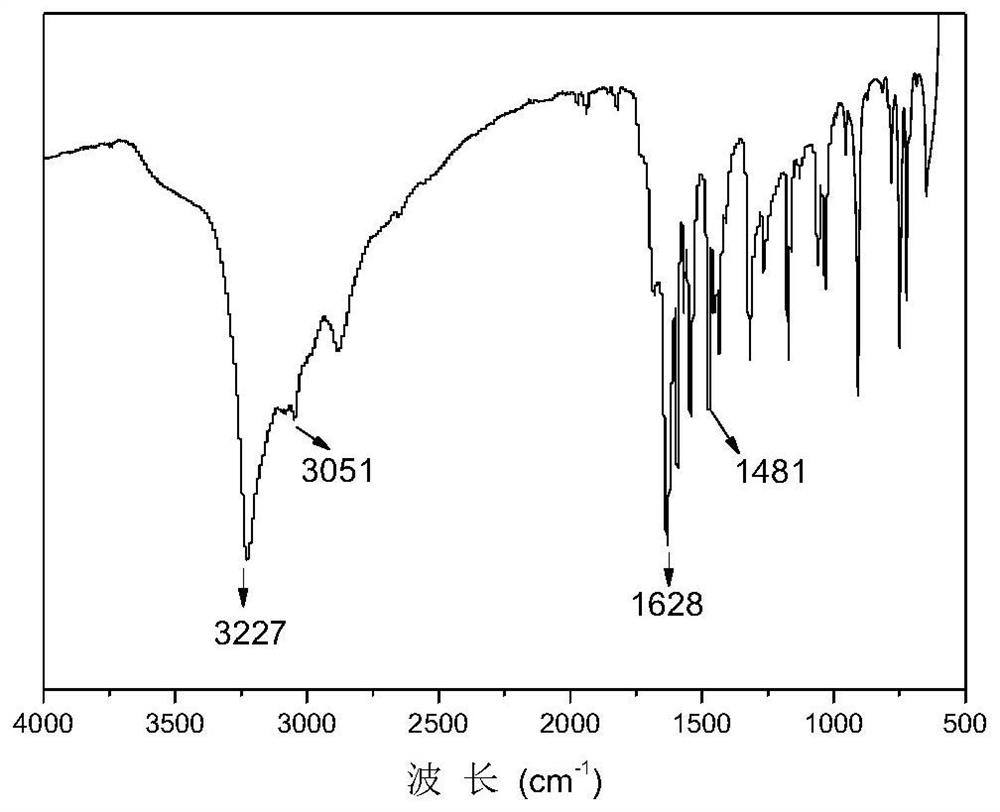
[0060] Mix the obtained ethyl 3,4-difluorobenzoate and hydroxylamine solution evenly, add sodium hydroxide ethanol solution, react at 50°C for 3 hours, add concentrated sulfuric acid, and obtain 3,4-difluorobenzhydroxime Acid, the structural formula is as follows, a total of 12.5g.
[0061]
Embodiment 3
[0063] This example provides a preparation method of perfluorobenzoic acid, the steps are: mix 10.0 g of perfluorobenzoic acid and 50 mL of butanol, then add 1.0 g of tere-benzenesulfonic acid catalyst, heat to 120 ° C, stir Reflux for 7 hours, and obtain perfluorobenzoic acid butyl fraction by vacuum distillation (120° C.);
[0064] Dissolve 3.6 g of hydroxylamine hydrochloride in 30 mL of chloroform solution, add sodium ethoxide, filter to remove the sodium chloride generated by the reaction, and obtain hydroxylamine solution;
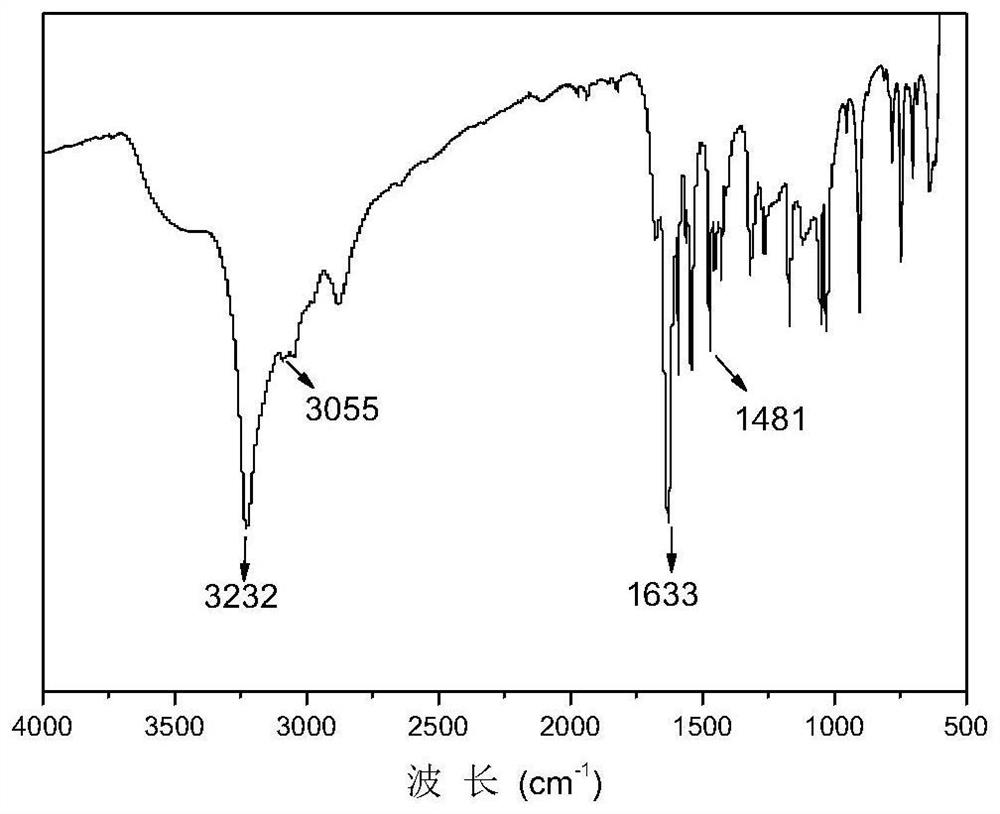
[0065] Mix the obtained butyl perfluorobenzoate and hydroxylamine solution evenly, add sodium hydroxide butanol solution, react at 50°C for 6 hours, add concentrated sulfuric acid, and obtain perfluorobenzoic hydroxamic acid, the structural formula is as follows, a total of 10.5 g.
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com