Application of non-metabolic function based on CHKalpha as target for cancer treatment, diagnosis and prognostic prediction
A technology for cancer treatment and metabolic function, applied in the field of oncology medicine, can solve problems such as inaccurate specific mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
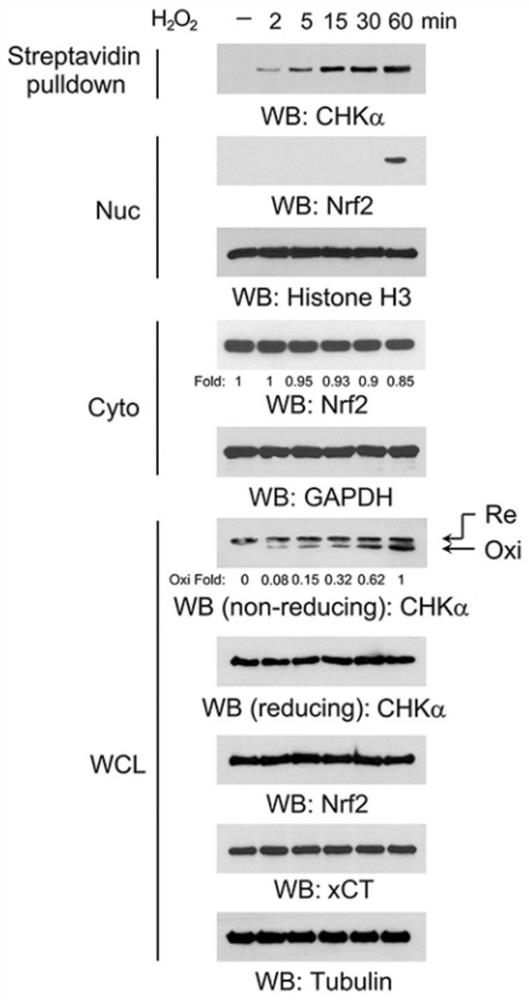
[0093] Example 1. CHKα is a direct sensor of oxidative stress
[0094] 1. The formation of intramolecular disulfide bonds in CHKα is a direct event for cells to respond to oxidative stress
[0095] with 50 μM H 2 o 2 Treatment of LN229 cells. Oxidized proteins were labeled with biotin-maleimide and purified with streptavidin-agarose beads. Such as figure 1 Shown, H 2 o 2 Intramolecular disulfide bonds were formed in CHKα at 2 minutes of treatment.
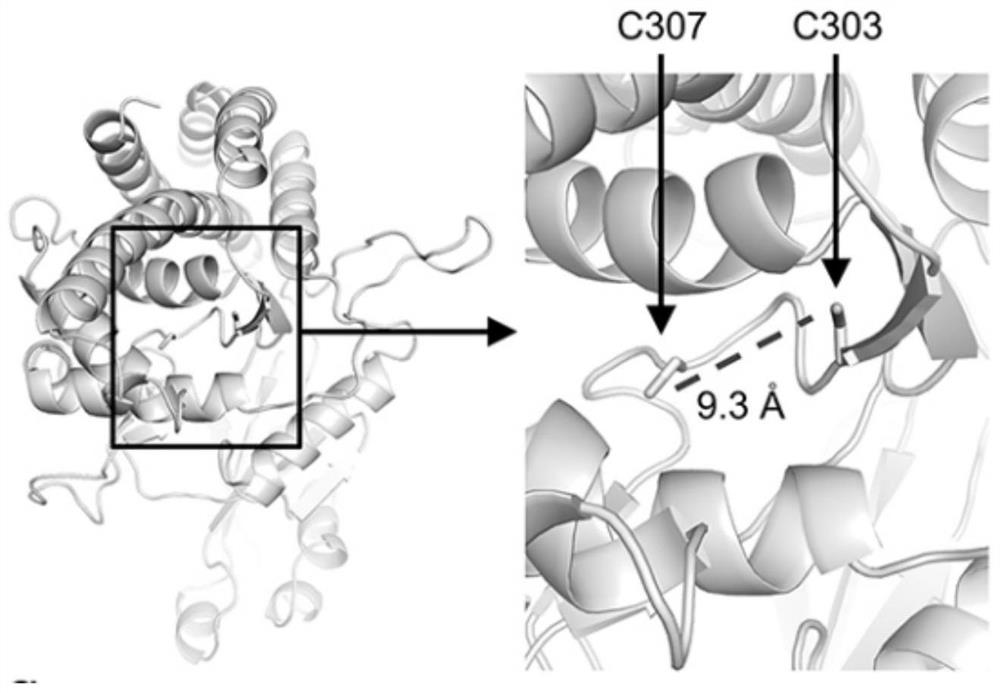
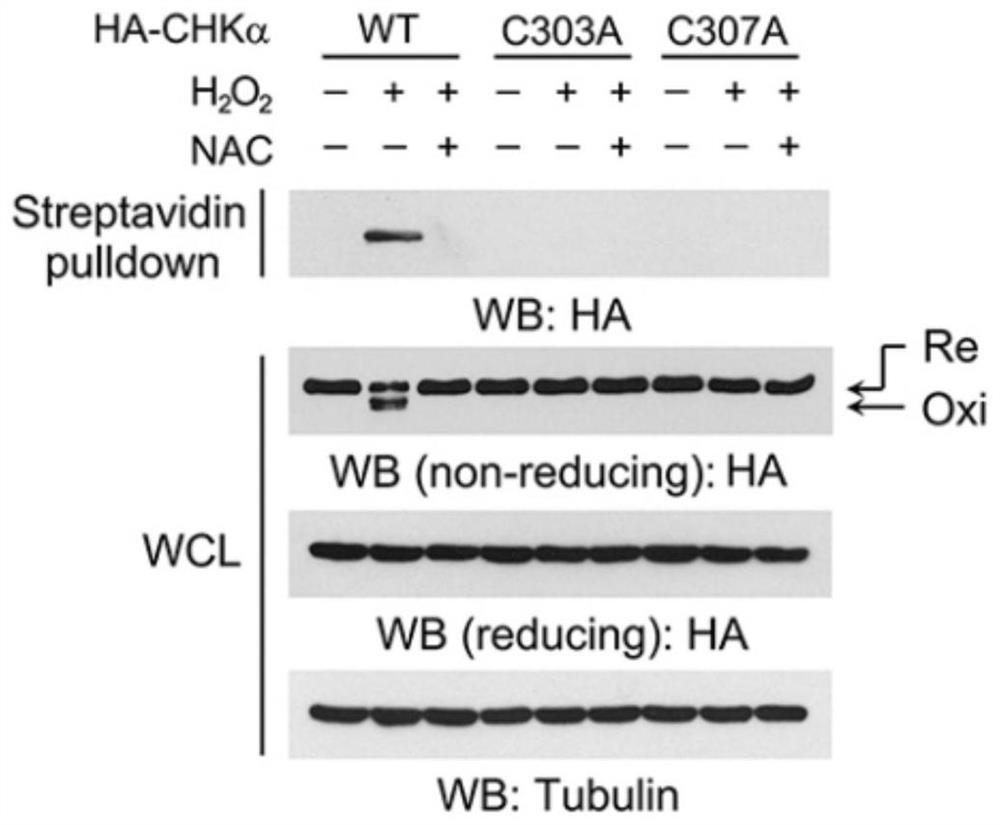
[0096] 2. The intramolecular disulfide bond of CHKα is formed between C303 and C307
[0097] (1) if figure 2 As shown, the human CHKα structure (PDB: 2CKO) shows the spatial location of C303 and C307. The surrounding areas of C303 and C307 are framed and magnified. An intramolecular disulfide bond is formed between the sulfur atoms of C303 and C307.
[0098] (2) Express HA-tagged wild-type CHKα, CHKαC303A, or CHKαC307A in LN229 cells. Cells were treated with the reducing reagent N-acetylcysteine (NAC) for 30 min, and...
Embodiment 2
[0099] Example 2. Oxidative stress causes CHKα to bind and phosphorylate CBS
[0100] 1.H 2 o 2 CHKα binds to CBS after treatment
[0101] LN229 cells expressing Flag-tagged CHKα and His-tagged CBS were treated with NAC for 10 minutes, and then treated with 50 μM H 2 o 2 Process for 10 minutes. Ni-NTA down experimental results show that H 2 o 2 Treatment resulted in binding of CHKα to CBS, which was blocked by NAC treatment. ( Figure 4 )
[0102] 2. Oxidative stress causes CHKα to phosphorylate the Y484 site of CBS
[0103] Purified wild-type Flag-CHKα was immobilized on magnetic beads, and added H 2 o 2 Treat for 10 minutes, rinse with PBS, and then incubate with purified wild-type His-CBS or His-CBS Y484F in the presence of 32P-ATP for in vitro kinase assay. The result is as Figure 5 , H 2 o 2 Treatment phosphorylates CBS, and the Y484F mutation of CBS blocks this phosphorylation.
Embodiment 3
[0104] Example 3, CHKα-mediated phosphorylation of CBS activates CBS
[0105] Purified wild-type Flag-CHKα or its mutant protein was immobilized on magnetic beads, and 50 μM H 2 o 2 Treat for 10 minutes, 10mM DTT for 30 minutes, rinse with PBS, and then incubate with purified wild-type His-CBS or His-CBSY484F in the presence of ATP for in vitro kinase assay (left) and CBS activity assay (right). The result is as Image 6 , Y484 phosphorylation of CBS enhances CBS activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com