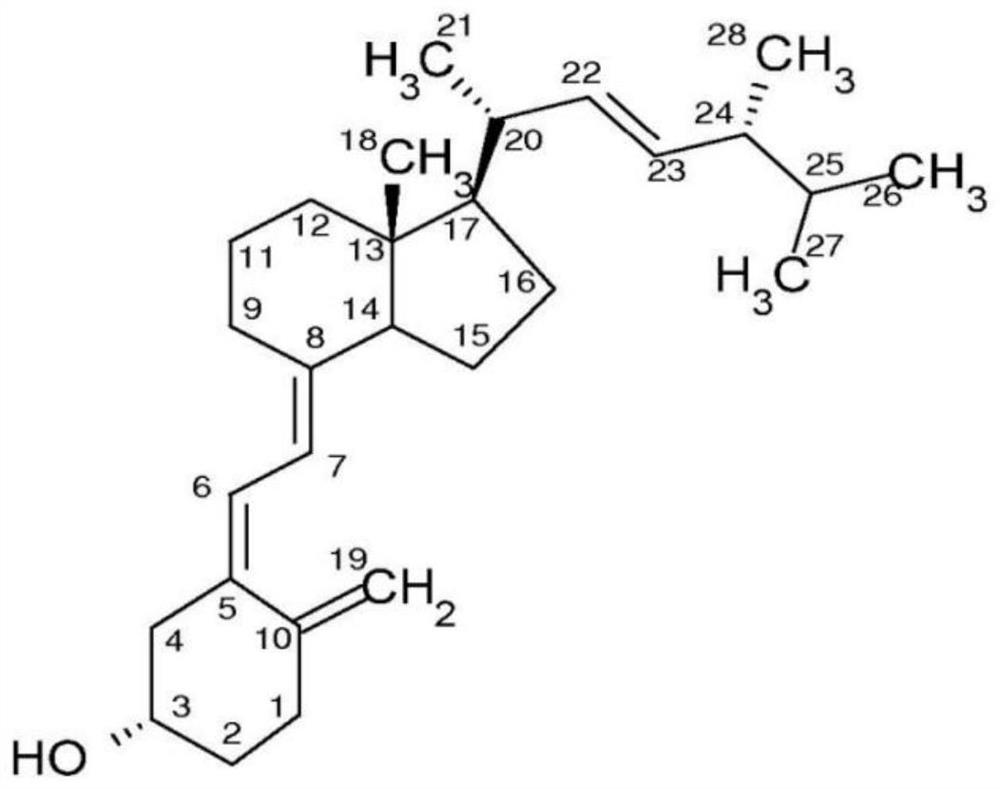
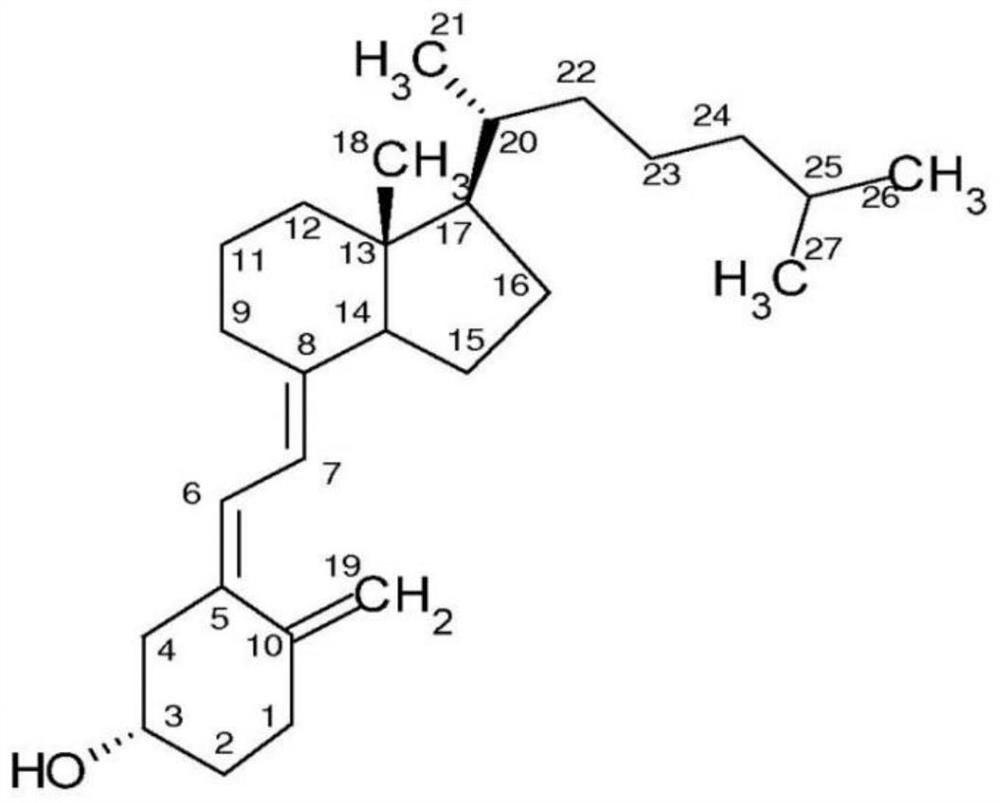
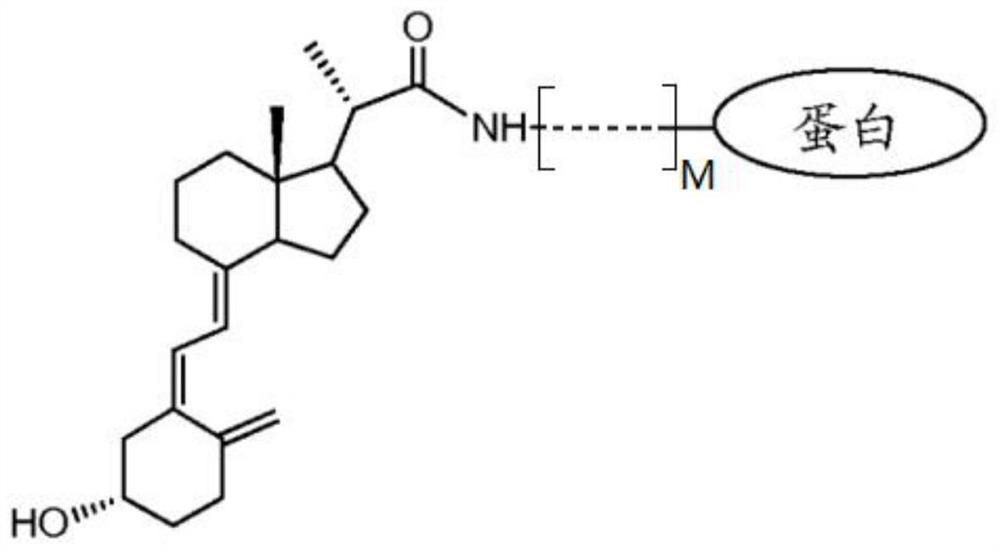
Immunodetection method of 25-hydroxyvitamin D and application of immunodetection method
An immunoassay method and hydroxyvitamin technology, which are applied in the field of immunoassays, can solve the problems of low detection sensitivity, high difficulty of hapten-conjugated markers, and great influence on product development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of 25-hydroxyvitamin D and macromolecular protein conjugates with two binding sites;
[0049] 1. Preparation of C3-position conjugates of 25-hydroxyvitamin D3 (purchased from Toronto Research Chemicals) and macromolecular protein (bovine serum albumin, purchased from Thermo);
[0050] Dissolve 1mg of 25-hydroxyvitamin D3 in an organic solvent (DMSO), add 10mg of succinic anhydride, under the protection of a suitable inert gas, react in the dark at room temperature for 12 hours, add the mixture to 20ml of ethyl acetate, and then Wash with ultrapure water, hydrochloric acid of appropriate concentration, ultrapure water successively, filter and vacuum dry to obtain 1mg 25-hydroxyvitamin D3 C3-hemisuccinate, dissolve in 1ml dichloromethane, add 0.2mg NHS and 0.4mg EDC , stirred at room temperature in the dark for 12 hours, filtered and vacuum-dried to obtain the activated ester of 25-hydroxyvitamin D3 C3-hemisuccinate, dissolved in 0.5ml organic solvent (DMSO) f...
Embodiment 2
[0054] ELISA indirect method to verify the activity of 25-hydroxyvitamin D and macromolecular protein conjugates with two binding sites;
[0055] 1. Dilute the above conjugate with coating solution (50mM / L carbonate buffer pH9.6) to 0.2-1μg / mL and add to 96-well microtiter plate (purchased from Xiamen Jincanhua), 100μL / well, 4 React overnight at ℃, wash the plate 3 times with washing solution (PBST); add 200 μL of blocking solution (1% bovine serum albumin in 10 mM / L pH7.4 phosphate buffer) to each well, incubate at 37 °C for 0.5 hours, wash with washing solution Wash the plate 3 times and dry it;
[0056] 2. Add an appropriate concentration of anti-25-hydroxyvitamin D antibody (the antibody comes from a number of IVD raw material companies) (diluted with blocking solution) 100 μL / well, incubate at 37°C for 0.5 hours, and wash the plate 3 times with washing solution;
[0057] 3. Add 100 μL / well of enzyme-labeled secondary antibody (purchased from Thermo, diluted with blocking...
Embodiment 3
[0062] Fluorescence lateral flow immunoassay method to screen antibody pairing raw materials for 25-hydroxyvitamin D detection kit;
[0063] Prepare the following components using conventional methods:
[0064] 1. Label the anti-25-hydroxyvitamin D 3 (C3) antibody and the anti-25-hydroxyvitamin D 22 (C22) antibody with fluorescein, and prepare the corresponding conjugate pads; select the following method as an example: Adjust the antibody concentration to 10 mg / ml with 0.02mol / LPBS (pH7.4); take FITC (purchased from Sigma Company) equivalent to 1 / 50 of the protein amount, and dissolve it in 0.2mol / L carbon dioxide equivalent to 1 / 10 of the volume of the protein solution. FITC was quickly added to the antibody under stirring conditions, and the reaction was stirred for 1 hour. After the labeling was completed, the fluorescein-labeled antibody was purified by dialysis, subpackaged and freeze-dried, and stored at -20°C for future use.
[0065] 2. Coating anti-25-hydroxyvitamin D ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com