Crystal form of aromatic ethylene derivative as well as preparation method and application of crystal form
A technology for crystal forms and compounds, which is applied to the crystal forms of aromatic vinyl derivatives and the fields of their preparation and application, can solve the problems of poor product stability, difficulty in production amplification, poor fluidity, etc., and achieves easy operation, high reproducibility, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
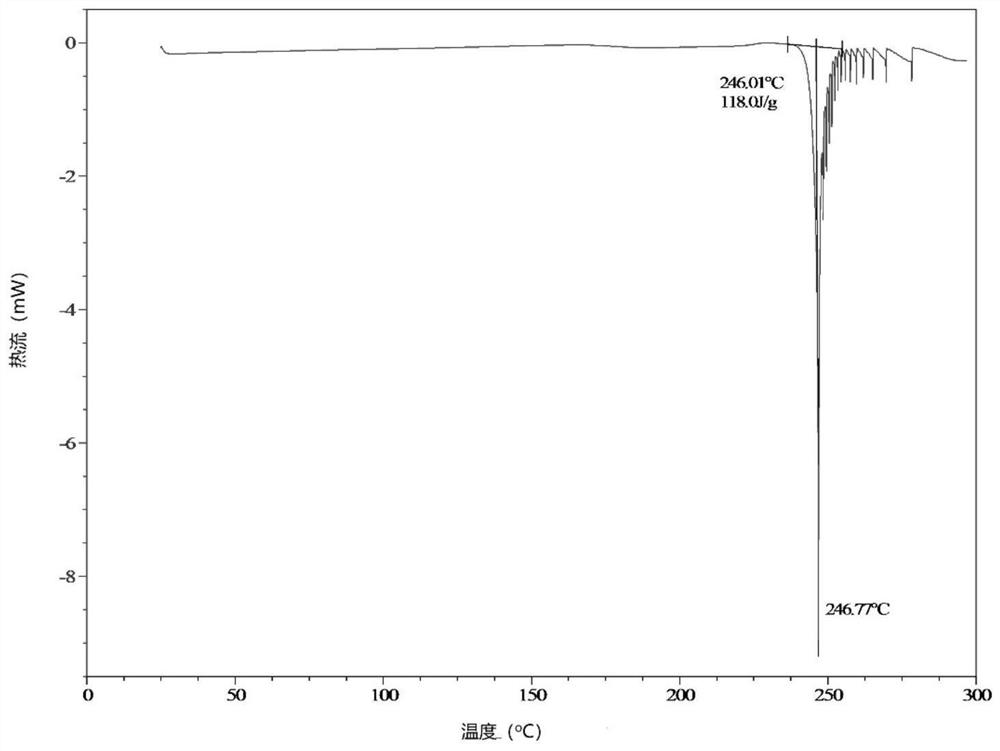
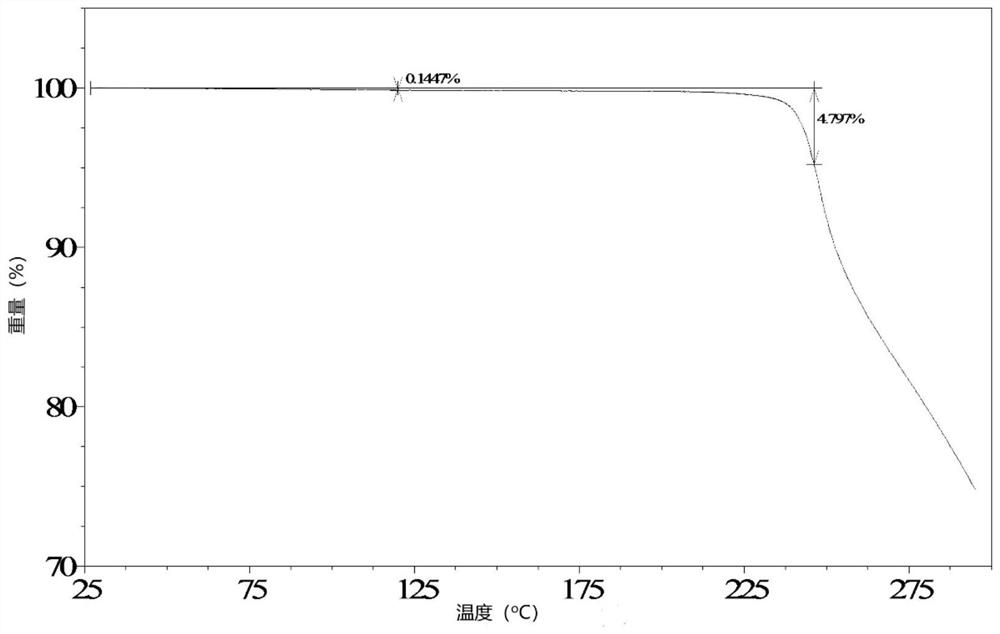
Image
Examples
preparation example Construction
[0111] In the preparation methods of the following crystal forms, the volume of added solvent (mL) = sample mass × volume multiple (mL), for example, the volume of ethanol in the preparation method 1 of Example 1 is: 0.02 × 50mL = 1mL.
[0112] Preparation of amorphous compound I (compound as shown in formula I):
[0113] Under room temperature conditions, methanol (10mL) and dichloromethane ( 10 mL) of the mixed solution was added glacial acetic acid (32.8 mg, 0.54 mmol), and the reaction solution was stirred at room temperature for 1 hour. Then, sodium cyanoborohydride (85.8 mg, 1.36 mmol) was added and stirred for 16 hours. The reaction solution was concentrated under reduced pressure, the residue was dissolved in ethyl acetate (50 mL), washed with water (20 mL) and saturated brine (20 mL), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was washed with dichloromethane : The mobile phase of methanol=10:1 was ...
Embodiment 1
[0115] Embodiment 1: the preparation method of crystal form A:
[0116] Preparation method 1: Weigh 20 mg of the compound shown in formula I into a 4 mL glass bottle, add 50 times the volume of ethanol (EtOH) into the glass bottle, and ultrasonically obtain a suspension of the sample for 1 minute, wrap the suspension sample vial Place the aluminum foil on a Labquaker rotator after shading from light, and start to rotate and balance at room temperature (about 20-25°C) 360 degrees. Samples were taken at 10 days and 20 days respectively, centrifuged, dried, and characterized by XRPD. The characterization result was crystal form A.
[0117] Preparation method 2: Weigh about 20 mg of the compound shown in formula I into a 10 mL glass vial, add 130 times the volume of ethanol (EtOH) into the glass vial, place the sample on a magnetic heating stirrer, and the temperature of the water bath is about 50 °C , rotate at 200rpm, heat to promote sample dissolution, keep warm for 15min, filt...
Embodiment 2
[0119] Embodiment 2: the preparation method of crystal form B:
[0120] Preparation method 1: Weigh 200 mg of the compound shown in formula I into a vial, add 10 times of ethanol and 40 times of water into the vial, stir and beat with magnetic force at room temperature for one day, centrifuge the solution, collect the solid, and dry at 40°C for 4 Hours, the dried solid was characterized, and the result of the characterization was crystal form B.
[0121] Preparation method 2: Weigh 100 mg of the compound shown in formula I into a small glass bottle, add 30 times the volume of water into the glass bottle, magnetically stir and beat for 17 days at room temperature, filter under reduced pressure, dry at 40°C for 4 hours, and pass XRPD Characterization, the characterization result is crystal form B.
[0122] Preparation method 3 (anti-solvent method): Weigh about 80 mg of compound samples of formula I into 20 mL glass vials, add an appropriate volume of good solvent to the vials ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| enthalpy of fusion | aaaaa | aaaaa |
| enthalpy of fusion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com