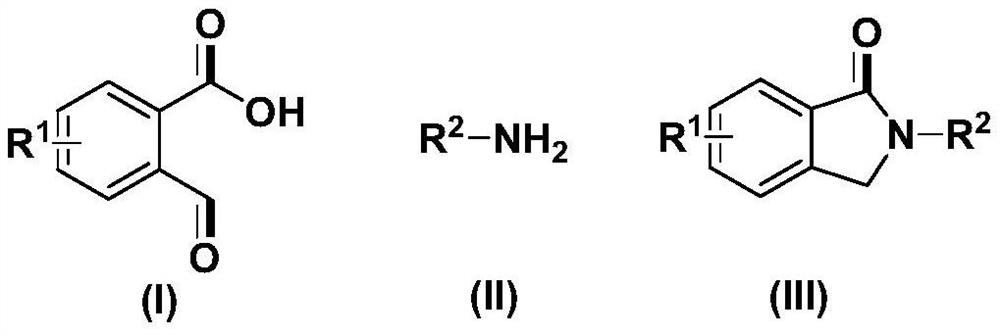
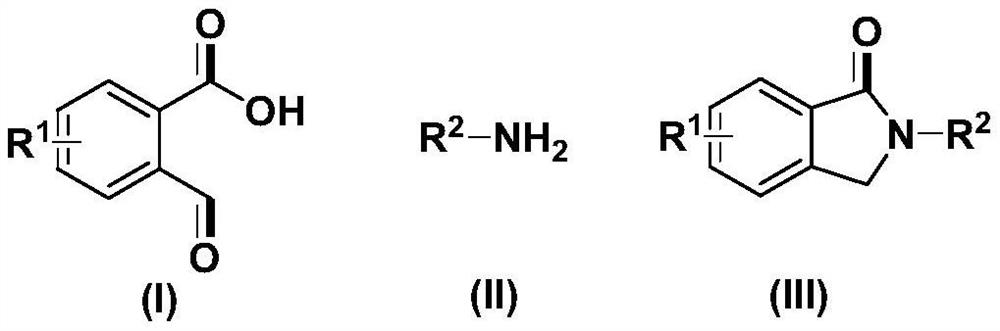
Method for preparing N-substituted pyrrolidone derivative
A technology for pyrrolidone and derivatives, which is applied in the field of preparing N-substituted pyrrolidone derivatives, can solve problems such as complex synthesis methods, and achieve the effects of simple operation, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
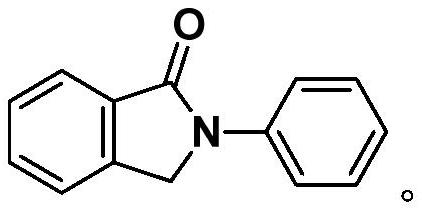
[0048] Example 1: Synthesis of 2-phenylisoindol-1-one:
[0049]
[0050] Take a three-neck round bottom flask, add 75mg (0.50mmol) of o-formylbenzoic acid, 70mg (0.75mmol) of aniline, 169.5mg (0.5mmol) of tetra-n-butyl ammonium hydrogensulfate, and 5mL of N,N-dimethylformamide, The carbon rod was used as the anode, and the carbon rod was used as the cathode, and the electrochemical reaction was carried out at room temperature (about 25° C.) and 10 mA current for 5 hours. After the reaction, add 20 mL of ethyl acetate and 100 mL of water for extraction, collect the organic phase after layering, and extract the water phase twice with ethyl acetate, each time the amount of ethyl acetate is 10 mL, combine the organic phases, add anhydrous sodium sulfate to dry , The solvent was distilled off under reduced pressure, and the product was obtained by column chromatography with a yield of 81%.
[0051] The characterization data for the product are:
[0052] 1 H NMR (500MHz, CDCl ...
Embodiment 2
[0055] Example 2: Synthesis of 2-(p-tolyl)isoindol-1-one:
[0056]
[0057] Take a three-neck round bottom flask, add 75mg (0.50mmol) of o-formylbenzoic acid, 107mg (1mmol) of p-methylaniline, 169.5mg (0.5mmol) of tetra-n-butyl ammonium hydrogensulfate, and N,N-dimethylformamide 5mL, with a carbon rod as the anode and a carbon rod as the cathode, electrochemically react at room temperature and 8mA for 5 hours. After the reaction, add 20 mL of ethyl acetate and 100 mL of water for extraction, collect the organic phase after layering, and extract the water phase twice with ethyl acetate, each time the amount of ethyl acetate is 10 mL, combine the organic phases, add anhydrous sodium sulfate to dry , The solvent was distilled off under reduced pressure, and the product was obtained by column chromatography with a yield of 83%.
[0058] The characterization data for the product are:
[0059] 1 H NMR (500MHz, Chloroform-d (CDCl 3 ))δ=7.95-7.91(m,1H),7.74(d,J=5.8Hz,2H),7.58(d...
Embodiment 3
[0062] Example 3: Synthesis of 2-(4-butylphenyl)isoindol-1-one:
[0063]
[0064] Take a three-neck round bottom flask, add 75mg (0.50mmol) of o-formylbenzoic acid, 112mg (0.75mmol) of p-n-butylaniline, 169.5mg (0.5mmol) of tetra-n-butyl ammonium hydrogensulfate, N,N-dimethyl Formamide 5mL, carbon rod as anode, carbon rod as cathode, electrochemical reaction at room temperature and 7mA current for 5 hours. After the reaction, add 20 mL of ethyl acetate and 100 mL of water for extraction, collect the organic phase after layering, and extract the water phase twice with ethyl acetate, each time the amount of ethyl acetate is 10 mL, combine the organic phases, add anhydrous sodium sulfate to dry , The solvent was distilled off under reduced pressure, and the product was obtained by column chromatography with a yield of 60%.
[0065] The characterization data for the product are:
[0066] 1 H NMR (500MHz, Chloroform-d) δ = 7.94 (dd, J = 7.2, 1.4Hz, 1H), 7.79-7.76 (m, 2H), 7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com