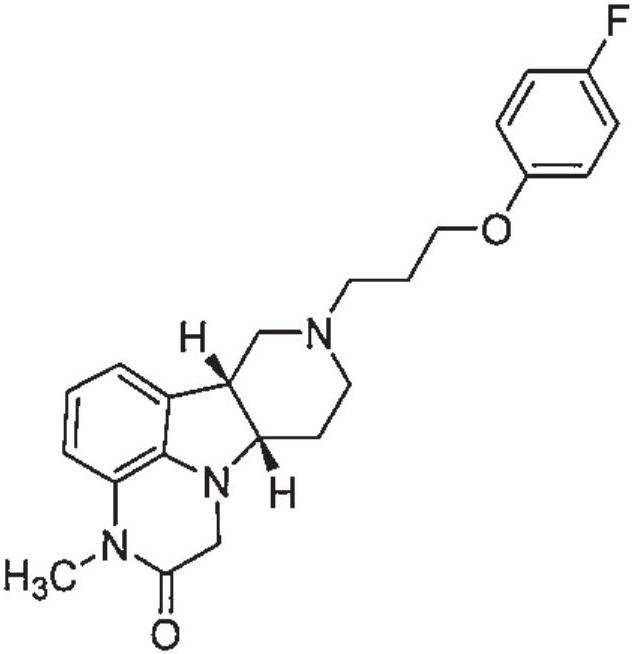
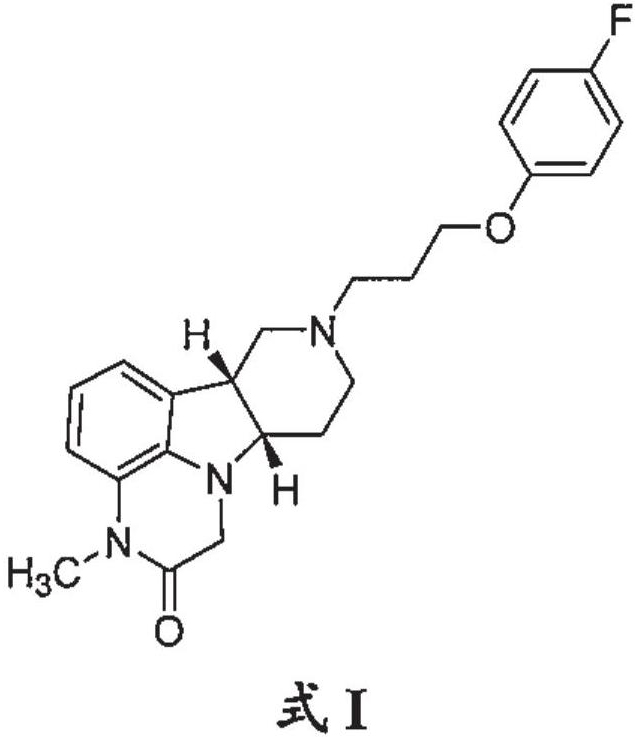
Organic compound
A technology of compounds and compositions, applied in organic chemistry, carbon-silicon compound conductors, organic active ingredients, etc., can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Example 1: (6bR,10aS)-8-(3-(4-fluorophenoxy)propyl)-3-methyl-6b,7,8,9,10,10a-hexahydro-1H-pyridine Synthesis of [3′,4′:4,5]pyrrolo[1,2,3-de]quinoxalin-2(3H)-one
[0194]
[0195] Sodium hydride (60% in mineral oil, 32 mg, 0.786 mmol) was added to (6bR, 10aS)-8-(3-(4-fluorophenoxy)propyl)-6b,7,8 at 0°C 9,10,10a-hexahydro-1H-pyrido[3′,4′:4,5]pyrrolo[1,2,3-de]quinoxalin-2(3H)-one (0.1g, 0.262 mmol) in DMF (1 mL). The mixture was stirred at 0°C for 30 minutes, and iodomethane (19.6 μL, 0.315 mmol) was added. The reaction mixture was stirred at 0°C for 1 hour, and water (4 mL) was added. The mixture was extracted with ethyl acetate (3×4ml) and washed over anhydrous Na 2 SO 4 The combined organic phases are dried. The mixture was filtered, and the filtrate was evaporated to dryness. The residue was purified by column chromatography using a 0-100% mixed solvent in ethyl acetate [ethyl acetate / methanol / 7N NH 3 (10:1:0.1 v / v)] eluted. The title compound (110 g, 99% y...
Embodiment 2
[0196] Example 2: 4-((6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido-[3′,4′:4,5]- Synthesis of pyrrolo[1,2,3-de]quinoxalin-8-(7H)-yl)-1-(4-fluorophenyl)-1-butanone
[0197]
[0198] (6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido-[3′,4′:4,5]-pyrrolo[1,2 , 3-de]quinoxaline (about 11.8g, about 50mmol), 4-chloro-4'-fluorobutyrophenone (15.0g, 74.8mmol), triethylamine (30mL, 214mmol) and potassium iodide (12.6g, 76 mmol) in dioxane (65 mL) and toluene (65 mL) was heated to reflux for 7 hours. After filtration and evaporation of the solvent, 200 ml of DCM were added. The DCM solution was washed with brine, dried (Na 2 SO 4 ) and concentrated to about 55ml. The concentrated solution was added dropwise to 600 ml of 0.5N HCl ether solution. The solid was filtered off, washed with ether, and dissolved in water. The resulting aqueous solution was basified with 2N NaOH and extracted with DCM. The DCM layers were combined, washed with brine (2×200 mL), dried (Na...
Embodiment 3
[0199] Example 3: (6bR,10aS)-8-(3-(4-fluorophenoxy)propyl)-6b,7,8,9,10,10a-hexahydro-1H-pyrido[3′, 4': Synthesis of 4,5]pyrrolo[1,2,3-de]quinoxalin-2(3H)-one
[0200]
[0201] (6bR,10aS)-6b,7,8,9,10,10a-hexahydro-1H-pyrido[3′,4′:4,5]pyrrolo[1,2,3-de]quinoxa Lin-2(3H)-one (100 mg, 0.436 mmol), 1-(3-chloropropoxy)-4-fluorobenzene (100 μL, 0.65 mmol) and KI (144 mg, 0.87 mmol) in DMF (2 mL) The mixture was degassed with argon for 3 min, and DIPEA (150 μL, 0.87 mmol) was added. The resulting mixture was heated to 78°C and stirred at this temperature for 2 hours. The mixture was cooled to room temperature, then filtered. The filter cake was purified by silica gel column chromatography using methanol / 7N NH 3 A gradient of 0-100% ethyl acetate in a mixture in methanol (1:0.1 v / v) as eluent gave a partially purified product, which was further analyzed with a semi-preparative HPLC system using 0.1% formic acid Gradient purification of 0-60% acetonitrile in water over 16 minutes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com