Ultra-long-acting controllable slow-release mesoporous-hyaluronic acid hybrid targeted antibacterial nano-material as well as preparation method and application thereof
A technology of hyaluronic acid and nanomaterials, applied in the direction of nanotechnology, nanomedicine, nanotechnology, etc., can solve the problems of short drug release time and uncontrollability, achieve excellent antibacterial effect, various forms, and prevent wound infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
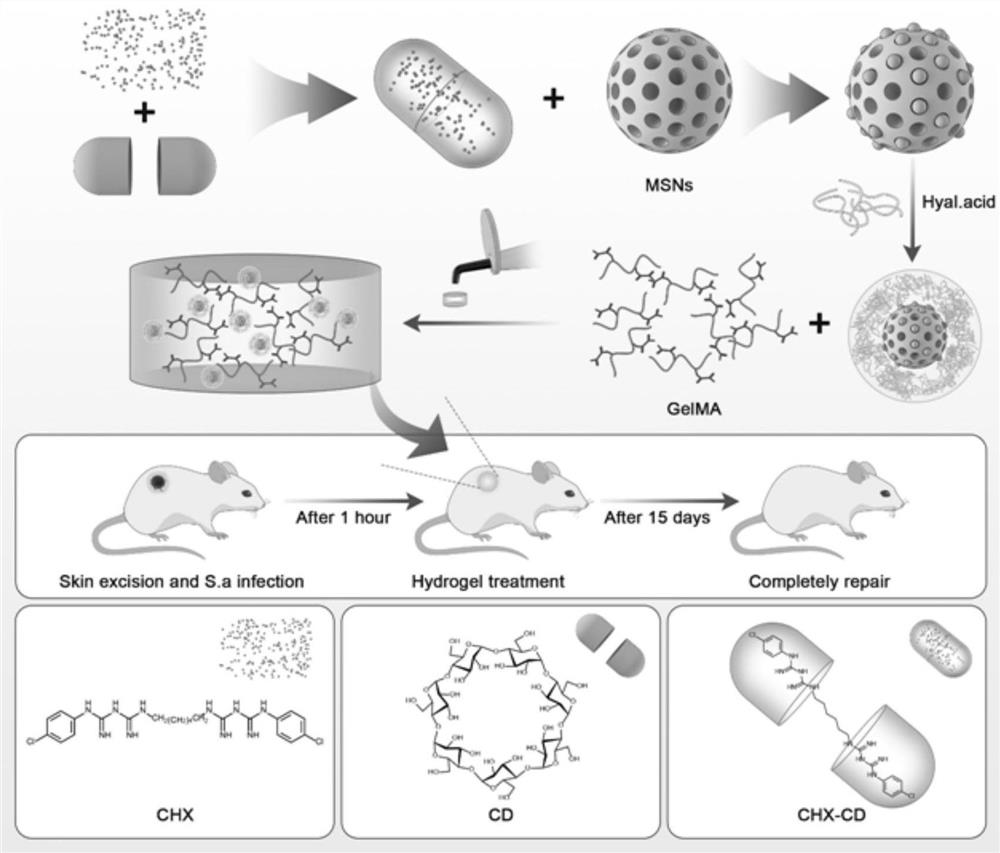
[0049] According to the examples disclosed in the present invention, the present invention exemplarily proposes a method for preparing an ultra-long-acting controllable sustained-release mesoporous-hyaluronic acid hybrid targeted antibacterial nanomaterial, which specifically includes the following steps:
[0050] First fully mix the acetone solution of the active drug with the cyclodextrin aqueous solution, filter and vacuum dry to obtain the clathrate;
[0051] Dissolving the clathrate in a buffer solution, mixing and reacting with the mesoporous silica nanoparticles dissolved in the buffer solution, to obtain a solution of mesoporous silica nanoparticles loaded with clathrates;
[0052] Then mix the hyaluronic acid dissolved in the buffer solution with the mesoporous silica nanoparticle solution loaded with clathrate, stir and centrifuge to obtain the first-generation mesoporous-hyaluronic acid hybrid nanoparticle;
[0053] Dissolving the first-generation mesoporous-hyaluro...
Embodiment 1
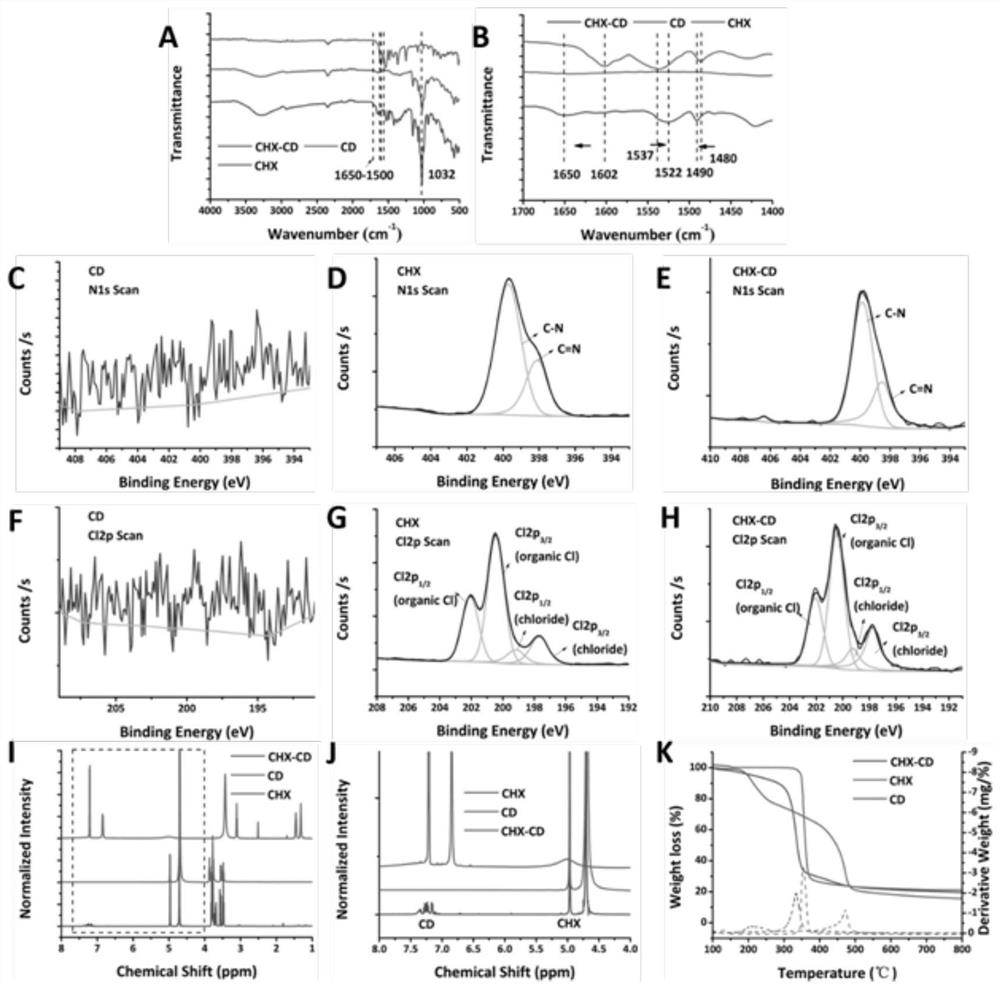
[0089] Synthesis of CHX-CD
[0090] Add 2.5mg / 10mL chlorhexidine acetone solution dropwise into 10mg / 10mL β-CD aqueous solution, wherein the volume ratio of the two is 1:1, and then fully mix and stir for 24 hours, after suction filtration and separation, vacuum drying to obtain chlorhexidine -Cyclodextrin inclusion complex (CHX-CD) is preserved for future use.
[0091]Characterization of CHX-CD
[0092] Fourier infrared:
[0093] Take an appropriate amount of sample (3.0mg), add potassium bromide powder (200mg), put it into an agate mortar under the irradiation of an infrared lamp, grind the two fully and evenly, and then transfer the ground fine powder to the matching tablet press In, a thin sheet with a thickness of about 0.5mm was made. Taking the blank disc as a control, the prepared thin slices were put into a Fourier transform infrared spectrometer for determination.
[0094] Proton NMR spectrum:
[0095] Dissolve the clathrate in D2O, prepare a solution with an ap...
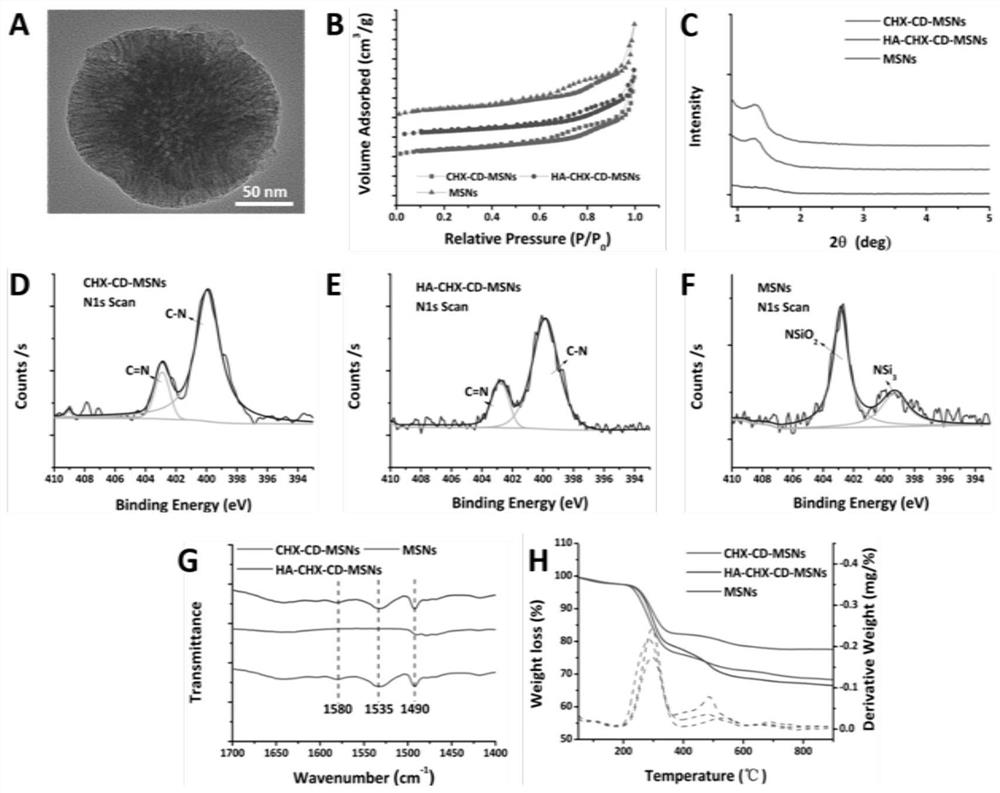
Embodiment 2
[0101] Preparation of HA-CHX-CD-MSNs
[0102] Dissolve 1.0g of CTAB in 480mL of deionized water, then add 3.5mL of 2mol / L NaOH, mix thoroughly, add 7.0mL of mesitylene, and stir vigorously in a water bath at 80°C for 2h.
[0103] Subsequently, 5.0 mL of TEOS was added dropwise, and the above temperature was kept at 80° C. and vigorously stirred for 2 h to form a white precipitate.
[0104] The product after the reaction was vacuum filtered, washed with a large amount of methanol solution, and dried overnight in vacuum, the initial product of MSN.
[0105] Take 1.0g of the dried material and disperse it in 100mL of methanol solution, then add 0.75mL of concentrated hydrochloric acid, stir in a 50°C water bath for 6h, and remove the template agent, mainly CTAB.
[0106] After the reaction was completed, they were filtered and washed by suction, dried in vacuum overnight, and used for later use to obtain MSNs.
[0107] Take 10 mg of the clathrate CHX-CD obtained in Example 1 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com