Artemisinin-base combination compound or pharmaceutically acceptable salt thereof, pharmaceutical preparation and application
A compound and artemisinin technology, applied in the field of medicine, can solve the problems of significant toxic and side effects, and achieve the effects of good drug resistance, good anti-tumor activity, and low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
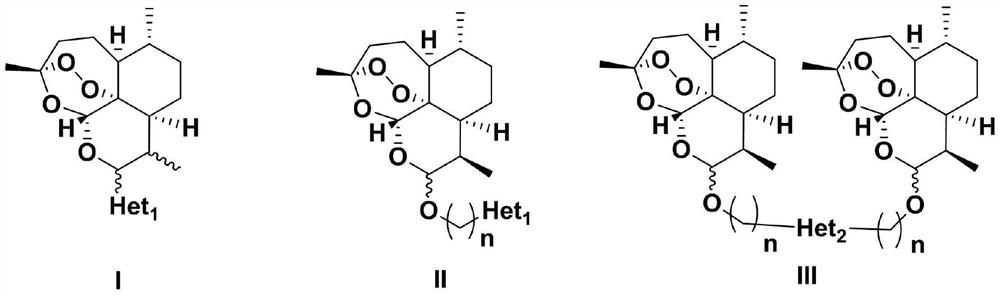
[0066] The main reaction scheme of the preparation method of artemisinin-base complex has:
[0067]
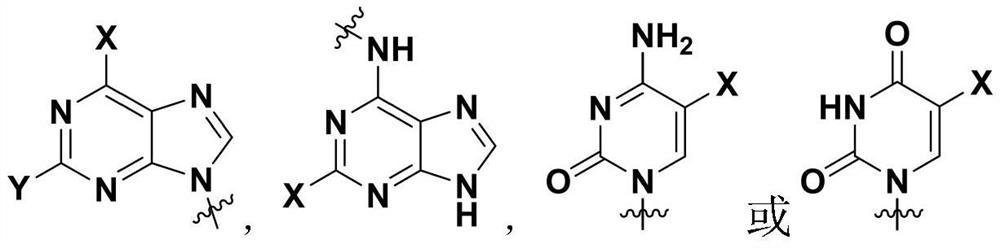
[0068] Het refers to Het as defined above 1 or Het 2 ;
[0069]
[0070] Among them, BF 3 ·Et 2 O is boron trifluoride diethyl ether, TFA is trifluoroacetic acid, Het 1 as defined above;
[0071]
[0072] DIAD is diisopropyl azodicarboxylate, TPP is triphenylphosphine, and X is as defined above.
Embodiment 1
[0073] Example 1: Preparation of 10-O-[2-(9H-9-purinyl)-ethyl]-(10S)-dihydroartemisinin
[0074] Step A: Preparation of 10-O-(2-bromoethyl)-(10S)-dihydroartemisinin
[0075]
[0076] Dissolve 5g (17.6mmol) of dihydroartemisinin (DHA) and 1.25mL (17.6mmol) of 2-bromoethanol in 100mL of refined THF (tetrahydrofuran), and slowly add 3.5mL (0.50g , 3.5mmol) boron trifluoride diethyl ether (BF 3 ·Et 2 O), stirred and reacted for 6 hours. The reaction process was monitored by thin layer chromatography (ethyl acetate:petroleum ether=1:4). After the reaction, add saturated NaHCO 3 solution. The organic layer was collected in layers, the aqueous layer was separated by extraction with ethyl acetate (25 mL×2), and the organic layers were combined. The organic layer was washed with 20 mL of saturated brine solution, and then washed with anhydrous Na 2 SO 4 After drying, the crude product was distilled off under reduced pressure to remove the solvent. The crude product was stat...
Embodiment 2
[0082] Example 2: Preparation of 10-O-[2-(6-amino-9H-9-purinyl)-ethyl]-(10S)-dihydroartemisinin
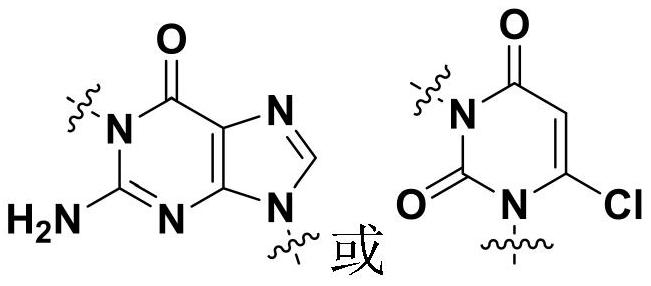
[0083] According to the preparation method of Example 1, the title compound was prepared by replacing the raw material 9H-purine in Example 1 with adenine;
[0084] LC-MS(m / z):446.2[M+H] +
[0085] 1 H NMR (CDCl 3 ,δ(ppm)):δ8.34(s,1H,Pur-H),7.88(s,1H,Pur-H),6.26(s,2H,-NH 2 ), 5.01 (s, 1H, H-12), 4.77 (d, J=3.5Hz, 1H, H-10), 4.59–4.46 (m, 1H, -OCH 2 C H 2 -),4.41–4.24(m,2H,-OC H 2 C H 2 -),3.76(ddd,J=10.2,6.5,3.6Hz,1H,-OC H 2 CH 2 -),2.63–2.57(m,1H,H-9),2.38–2.30(m,1H,H-4),1.42(s,3H,H-14),0.94(d,J=6.2Hz,3H , H-16), 0.80 (d, J=7.4Hz, 3H, H-15).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com