Thioetherified aromatic heterocyclic compound as well as preparation method and application thereof
A technology for aromatic heterocycles and compounds, which is applied in the field of thioetherified aromatic heterocycles and their preparation, can solve problems such as unfavorable industrial production, increase the difficulty and cost of obtaining reaction raw materials, and achieves low cost, easy industrialization, and substrates. Wide choice of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
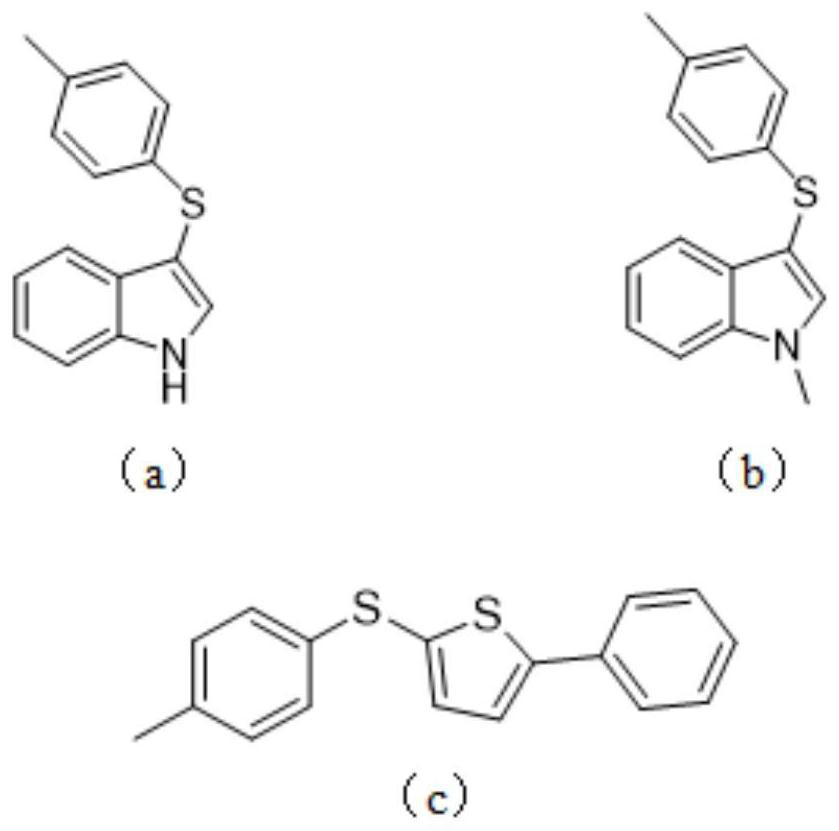
[0036] A kind of thioetherification aromatic heterocyclic compound, its molecular structural formula is as follows figure 1 Chinese formula (a) shown.
[0037] The preparation method of the above-mentioned thioetherated aromatic heterocyclic compound comprises the following steps:
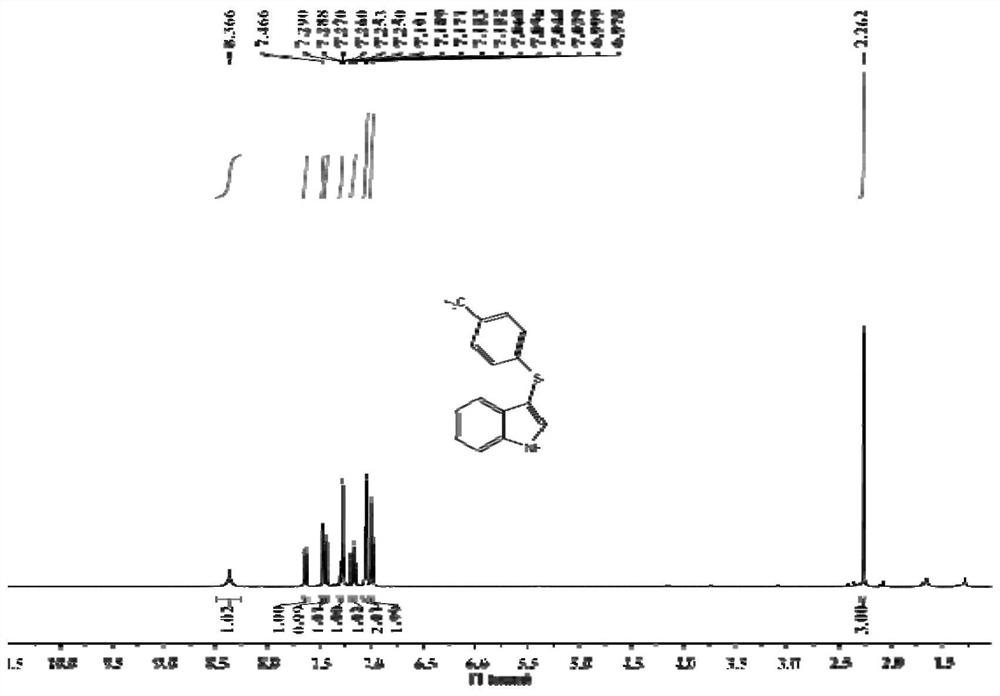
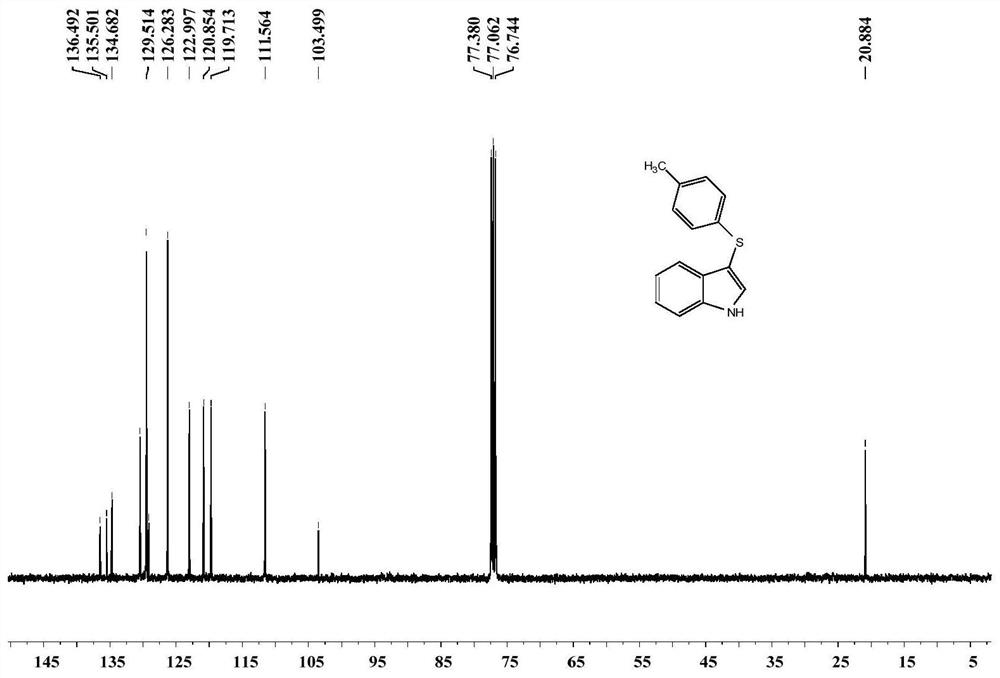
[0038] Take a 15mL pressure-resistant reaction tube, add PIFA 90mg, indole 24mg, 4-methylthiophenol 30mg, dichloromethane 2mL, open the reaction, and stir overnight at 30°C. After the reaction, add 10 mL of ethyl acetate to quench the reaction, add 10 mL of brine to wash, separate the organic phase, extract the aqueous phase with ethyl acetate three times, combine the organic phases, and separate by column chromatography to obtain 3-(p-tolylthio)- 38.4 mg of pure 1H-indole, yield 80%.
Embodiment 2
[0040] Different from Example 1, this example uses acetonitrile instead of dichloromethane. The yield of 3-(p-tolylthio)-1H-indole in this example was 50%.
Embodiment 3
[0042] Different from Example 1, this example uses Selectfluor fluorine reagent instead of PIFA. The yield of 3-(p-tolylthio)-1H-indole in this example was 45%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap