A kind of polyester block copolymer, its one-pot synthesis method and application
A technology of block copolymers and polyesters, applied in the field of polymer electrolytes for lithium ion batteries, can solve the problems of complex preparation methods and uncontrollable reactions, and achieve the advantages of enhancing room temperature ions, overcoming low mechanical strength, and overcoming catalyst metal residues. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
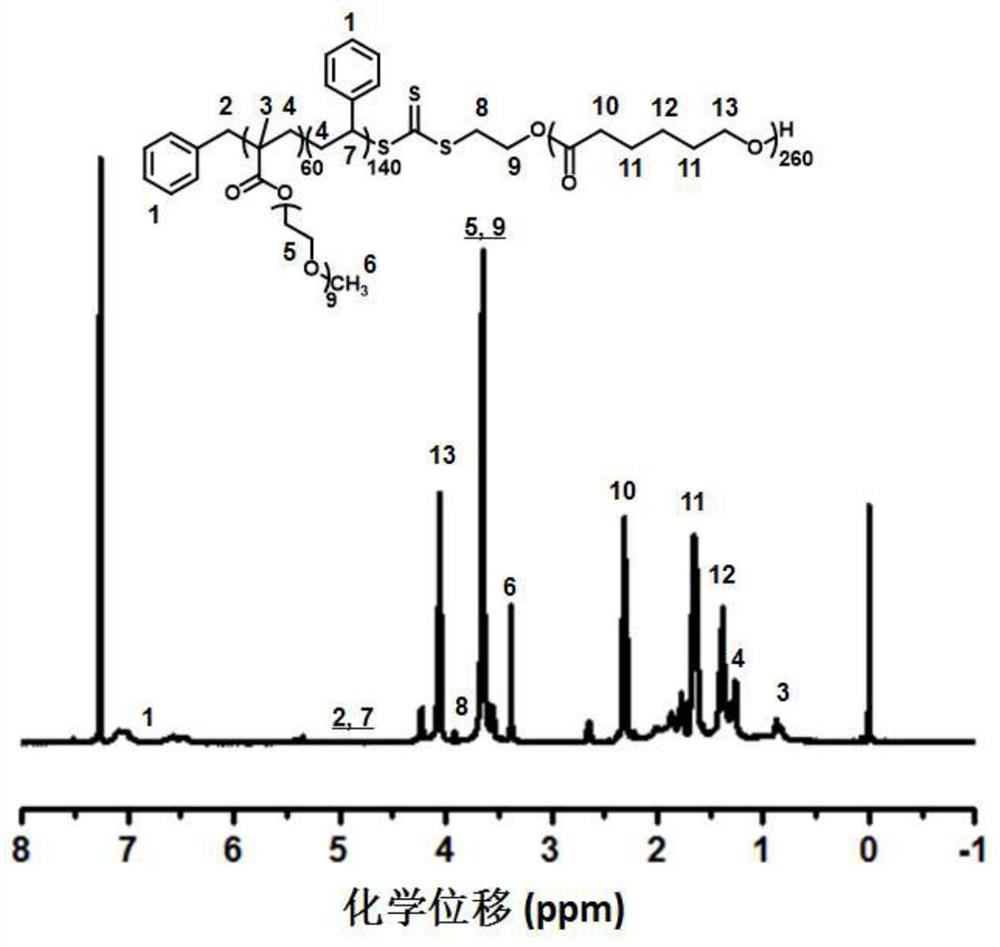
[0046] A kind of polyester block copolymer base polymer electrolyte has the chemical structure of following formula:
[0047]
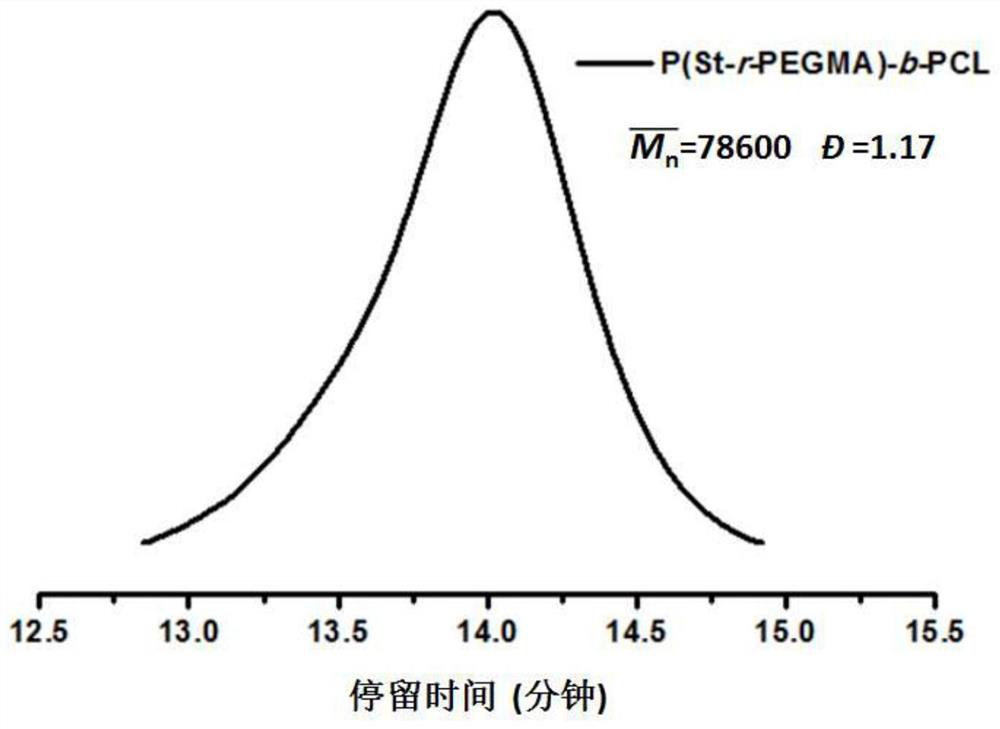
[0048] The preparation of the polyester block polymer-based electrolyte, such as figure 1 shown, including the following steps:
[0049] (1) Under anhydrous and oxygen-free conditions, 1.8g of styrene, 4.15g of ε-caprolactone, and 3.4g of poly(ethylene glycol) methacrylate monomers were uniformly mixed to form a monomer mixture;
[0050] (2) Add 11.21mg of 4,4'-azobis(4-cyanovaleric acid) and 29.33mg of 2-(benzylsulfanylthiocarbonylsulfanyl)ethanol to the monomer mixture and mix well;
[0051] (3) Raise the raw material mixture to 50°C for 36 hours in an argon atmosphere to allow reversible addition-fragmentation transfer polymerization of vinyl monomers, and then raise the temperature to 110°C for 48 hours to allow ring-opening polymerization of cyclic lactones . After the reaction is completed, the reaction is cooled to room temperature, and t...
Embodiment 2
[0058] A kind of polyester block copolymer base polymer electrolyte has the chemical structure of following formula:
[0059]
[0060] The preparation of the polyester block polymer-based electrolyte comprises the following steps:
[0061] (1) Under anhydrous and oxygen-free conditions, 2.50g styrene, 4.9g δ-valerolactone, and 2.8g poly(ethylene glycol) methacrylate monomers were uniformly mixed to form a monomer mixture;
[0062] (2) Add 11.21mg of 4,4'-azobis(4-cyanovaleric acid) and 29.33mg of 2-(benzylsulfanylthiocarbonylsulfanyl)ethanol to the monomer mixture and mix well;
[0063] (3) Raise the raw material mixture to 60°C for 30 hours in an argon atmosphere to allow reversible addition-fragmentation transfer polymerization of vinyl monomers, and then raise the temperature to 120°C for 40 hours to allow ring-opening polymerization of cyclic lactones . After the reaction is completed, the reaction is cooled to room temperature, and the reaction is quenched to obtain ...
Embodiment 3
[0069] A kind of polyester block copolymer base polymer electrolyte has the chemical structure of following formula:
[0070]
[0071] The preparation of the polyester block polymer-based electrolyte comprises the following steps:
[0072] (1) Under anhydrous and oxygen-free conditions, uniformly mix 2.14g styrene, 10.6g DL-lactide, and 2.24g poly(ethylene glycol) methacrylate monomers to form a monomer mixture;
[0073] (2) Add 11.21mg of 4,4'-azobis(4-cyanovaleric acid) and 29.33mg of 2-(benzylsulfanylthiocarbonylsulfanyl)ethanol to the monomer mixture and mix well;
[0074] (3) Raise the temperature of the raw material mixture to 70°C for 24 hours in an argon atmosphere to allow reversible addition-fragmentation transfer polymerization of vinyl monomers, and then raise the temperature to 130°C for 32 hours to allow ring-opening polymerization of cyclic lactones . After the reaction is completed, the reaction is cooled to room temperature, and the reaction is quenched t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com