Aeromonas salmonicida and edwardsiella tarda bivalent vaccine and application thereof
A technology for killing Aeromonas salmonicum and Edwardsiella tarda, which is applied in the field of Aeromonas salmonicum killing and Edwardsiella tarda dual vaccine, can solve the problems of waste of manpower and material resources, no turbot scabies and ascites diseases, etc. , to reduce environmental hazards, eliminate residual hidden dangers, and enhance immune response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Isolation and identification of Aeromonas salmonicida and E. tarda embodiment
[0030] 1 strain
[0031] Aeromonas salmonicida and tarda, from a farm in Shandong diseased fish separate.
[0032] 2, method
[0033] 2.1 Screening of vaccine strains
[0034] The prevalence of a turbot farm from Yantai, Shandong, diseased fish mainly for the abdominal part of the red area, body surface ulcers, liver local redness. With saline diseased turbot washed three times, the removal of kidney tissue of diseased fish in aseptic technique. Normal saline tissue block is placed in a sterile 2.0ml centrifuge tube, cut into pieces and a small amount streaked on TSB plates, 28 deg.] C incubation 24h. After colonies grow, the color of the same size were picked single colonies were identified. After delivery company sequenced, and the resulting sequence homology in the NCBI site sequence alignment analysis.
[0035] 2.2 Identification of results
[0036] 1, the results of morphological ...
Embodiment 2 2
[0044] Example 2 Preparation of two vaccines
[0045] After the above-mentioned murderous mono cyclasmia is activated, the scribe is inoculated on the LB solid petriper, 28 ° C culture 24 h, and picks up a single bacterius to the LB liquid medium culture for 24 h, and send the company to sequencing and identifying. After the monohydrococcus, the bacterium in the medium was collected from centrifugation at 4 ° C, and the precipitate was cleaned by sterile PBS (pH 7.3), and finally containing 0.5% formaldehyde (V / V). The PBS was resuspended and placed at 4 ° C for 24 h. The next day, a small portion of the solution was washed three times with a sterile PBS. After the coating plate was determined, the precipitate was collected by centrifugation (4 ° C) at 5000 r / min (4 ° C), removed, and washed 3 times with Sterilization. , And heavy suspension, the concentration is about 1 × 10 9 CFU / mL, the resulting solution is a mild splita vaccine. The same method was used to get slowed Ed...
Embodiment 3
[0047] Example 3 Immune animal experiment
[0048] 1, immunization method
[0049] Choose 300 tail healthy tailings, divided into vaccine groups and control groups, each group of 150 tail fish. Immunization in the abdominal injection. The vaccine group is injected with a concentration of 0.1 ml of a concentration of 1 × 10, respectively. 9 The inactivated vaccine of CFU / mL was injected with 0.1 ml of sterile PBS in the control group.
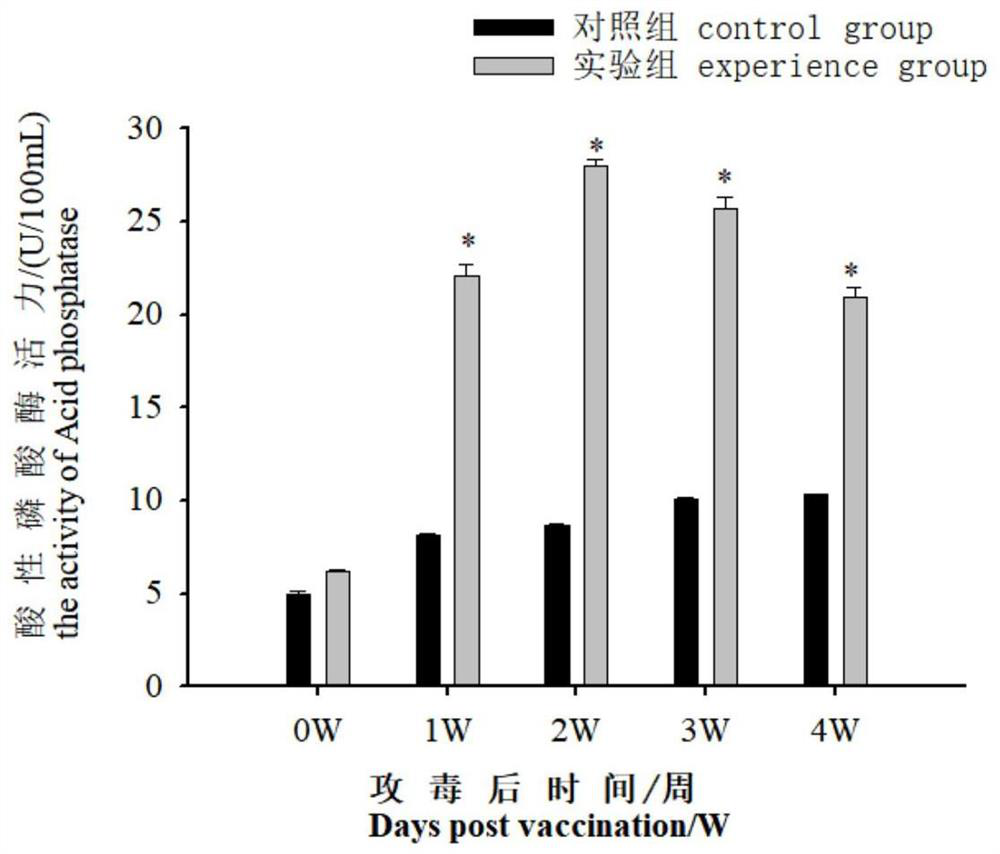
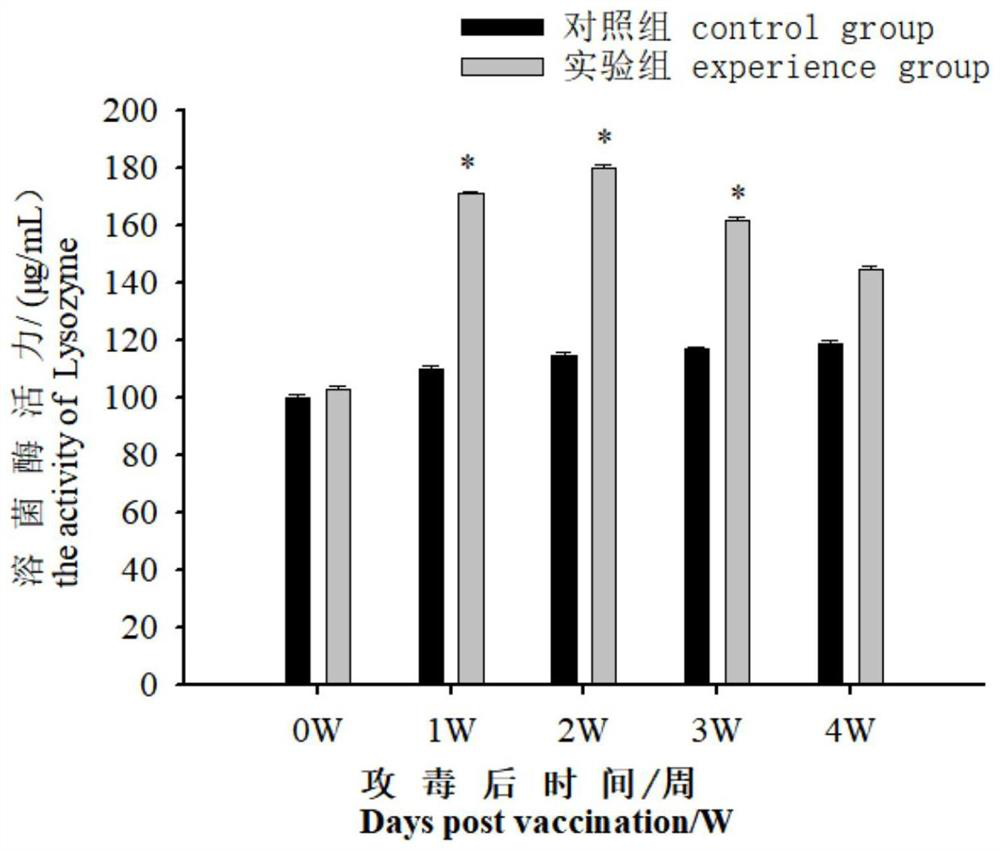
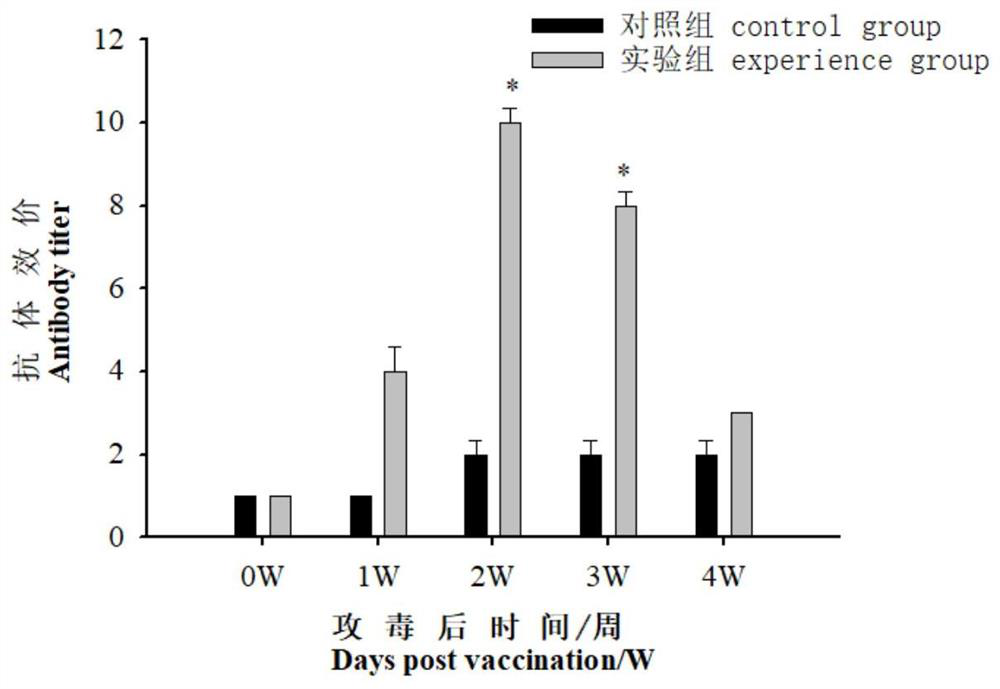
[0050] 2. Determination of acid phosphatase (ACP) and lysozyme (LZM) vitality during immunized serum
[0051] The blood samples were performed on Daxue (1, 2, 3, 4w) before immunization (1, 2, 3, 4w), each of which were randomly taking 9 tail fish each time. After standing at room temperature for 2 h, 4 ° C, 3000r / min centrifuge 15min, take the supernatant, according to the corresponding step of the kit (Nanjing Implementation Biological Engineering Research Institute), using the kit to measure the acid phosphatase in serum Activity to lysozyme....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com