Iron-based Prussian blue, and preparation method and application thereof
A Prussian blue, iron-based technology, used in ferricyanide, structural parts, metal cyanide, etc., can solve the problems of insufficient specific energy of electrode materials, restricting the large-scale application of materials, and easy to release toxic substances, and achieve safe production process. Non-toxicity, cycle stability, improved charge-discharge specific capacity, and fewer product defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
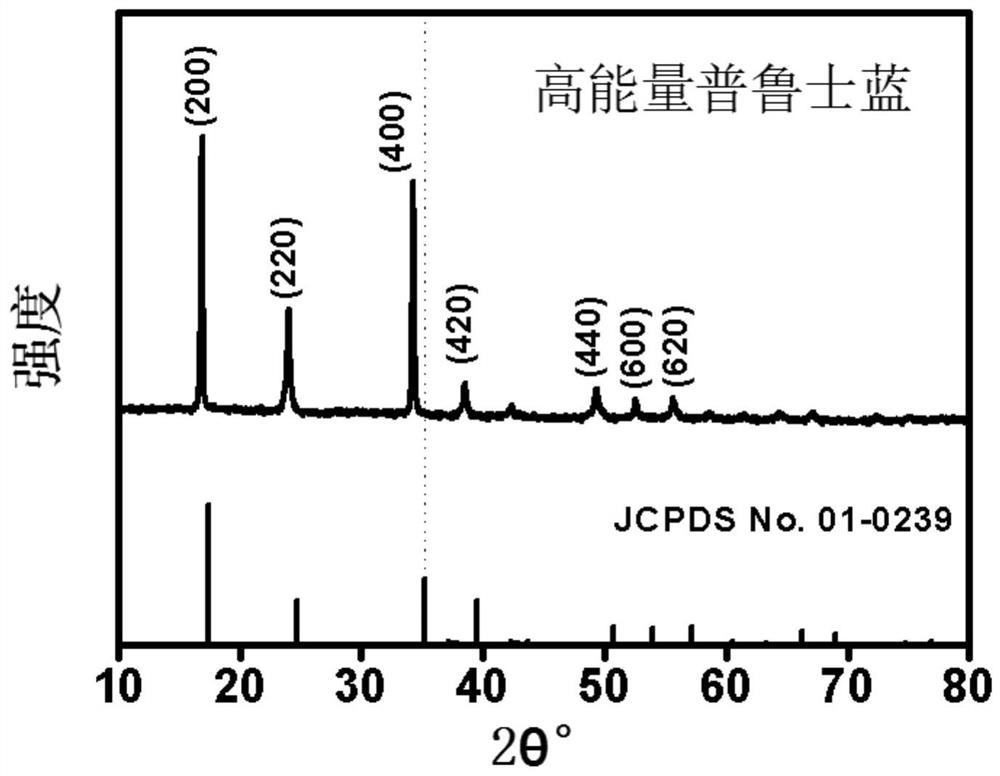
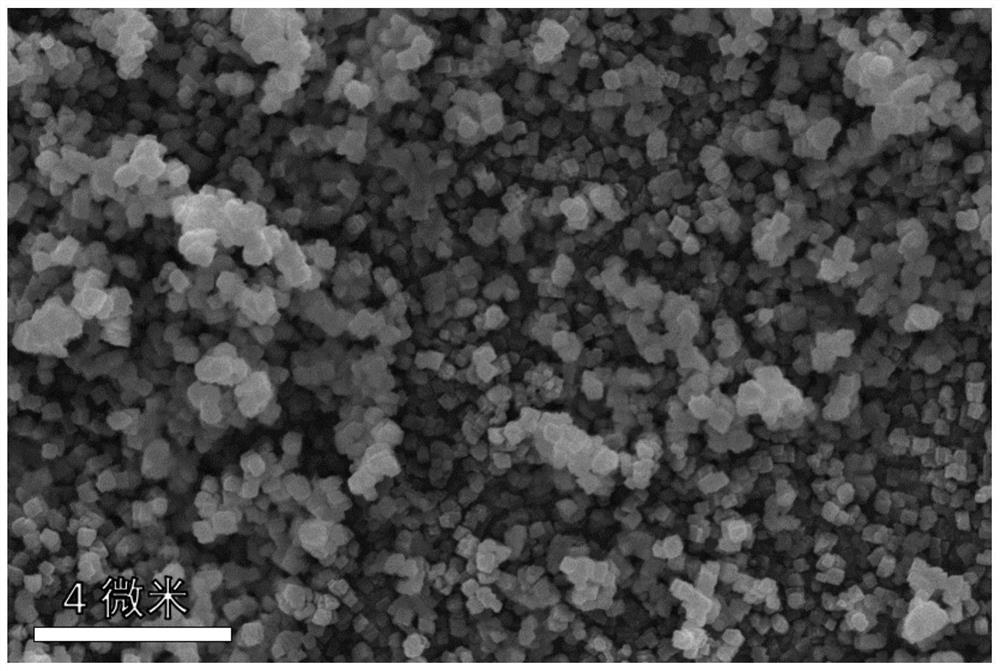
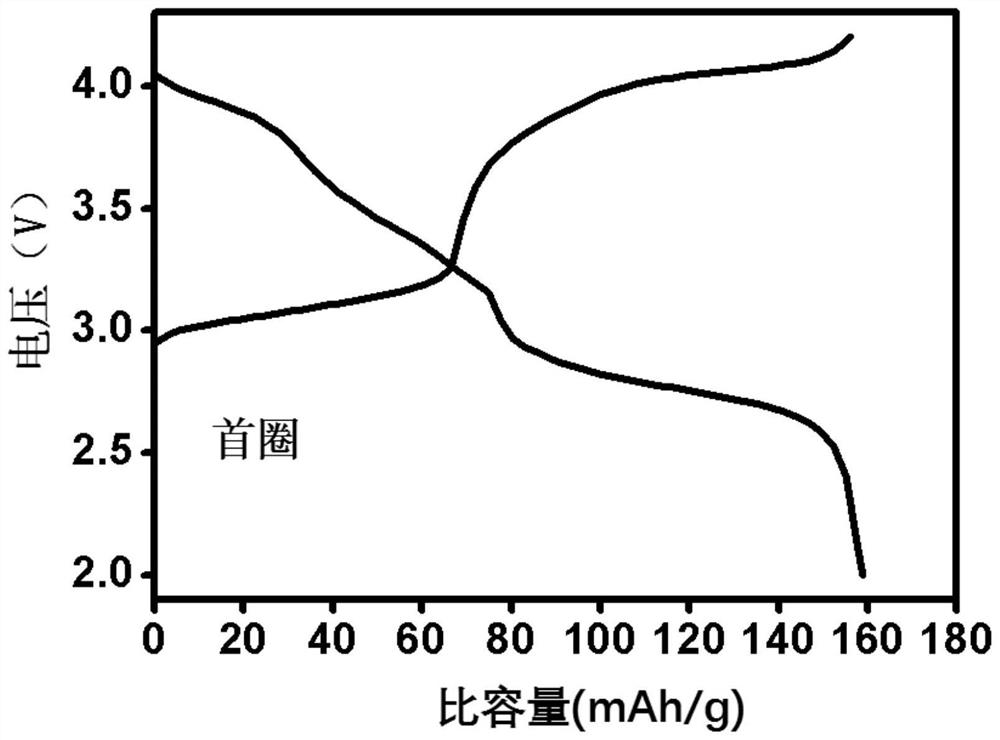
[0032] This embodiment provides a preparation method of iron-based Prussian blue, the iron-based Prussian blue prepared by the preparation method and the iron-based Prussian blue as a positive electrode material for a sodium ion battery. The method comprises the steps of:
[0033] Add 48.4 grams of sodium ferrocyanide powder with a purity greater than 99.5% into 100 ml of deionized water, and stir at 40°C until a nearly saturated yellow solution of sodium ferrocyanide is formed 1;
[0034] S2 Add 0.556 g of ferrous sulfate powder with a purity greater than 99.5% and 5 g of trisodium citrate into 100 ml of deionized water, and stir until a clear solution 2 is formed;
[0035] In S3, the solution 1 obtained in the above steps is directly poured into the solution 2, and the mixed solution is continuously stirred for 0.5 hours, and then left to stand at a temperature of 25°C for 24 hours to obtain a light blue precipitate;
[0036] S4 collects the blue precipitate obtained in ste...
Embodiment 2
[0041] This embodiment provides a preparation method of iron-based Prussian blue, the iron-based Prussian blue prepared by the preparation method and the iron-based Prussian blue as a positive electrode material for a sodium ion battery. The method comprises the steps of:
[0042] Add 48.4 grams of sodium ferrocyanide powder with a purity greater than 99.5% into 100 ml of deionized water, and stir at 40°C until a nearly saturated yellow solution of sodium ferrocyanide is formed 1;
[0043] S2 Add 0.446 grams of manganese sulfate powder with a purity greater than 99.5% and 5 grams of trisodium citrate into 100 milliliters of deionized water, and stir until a clear solution 2 is formed;
[0044] In S3, the solution 1 obtained in the above steps is directly poured into the solution 2, and the mixed solution is continuously stirred for 0.5 hours, and then left to stand at a temperature of 25° C. for 24 hours to obtain a white precipitate;
[0045] S4 collects the blue precipitate...
Embodiment 3
[0060] This embodiment provides a preparation method of iron-based Prussian blue, the iron-based Prussian blue prepared by the preparation method and the iron-based Prussian blue as a positive electrode material for a sodium ion battery. The method comprises the steps of:
[0061] Add 30 grams of sodium ferrocyanide powder with a purity greater than 99.5% into 100 milliliters of deionized water, and stir at a temperature of 40°C until a nearly saturated yellow solution 1 of sodium ferrocyanide is formed; the salt solution 1 has a concentration of 0.62 mol / L nearly saturated solution;
[0062] S2 Add 0.556 g of ferrous sulfate powder with a purity greater than 99.5% and 5 g of trisodium citrate into 100 ml of deionized water, and stir until a clear solution 2 is formed;
[0063] In S3, the solution 1 obtained in the above steps is directly poured into the solution 2, and the mixed solution is continuously stirred for 0.5 hours, and then left to stand at a temperature of 25°C f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Scale | aaaaa | aaaaa |
| Quantity density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com