Preparation method of eye drops
A technology of eye drops and solutions, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of poor water solubility and increase drug selectivity, and achieve high water solubility, Effect of increasing drug selectivity and strong hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
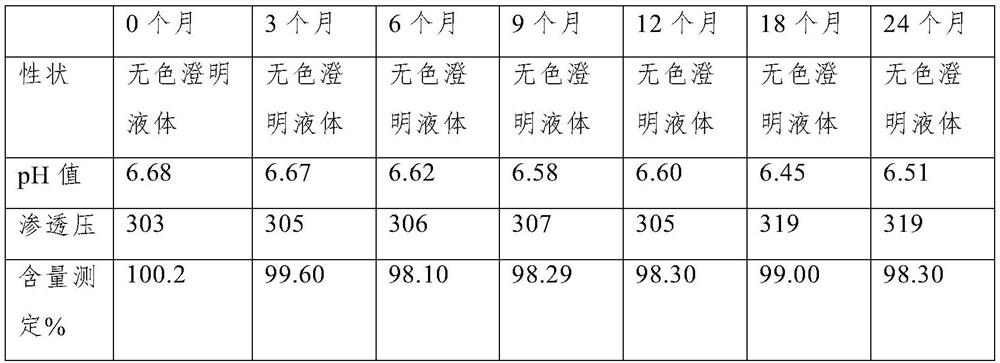
[0029] This embodiment provides a preparation method of eye drops, the osmotic pressure of the eye drops is 303mOsm / L, the eye drops include voriconazole solution complexed with sulfobutyl-β-cyclodextrin, the drops The content of voriconazole in eye drops is 4mg / mL;
[0030] Described preparation method comprises:
[0031] Step 1, 4g voriconazole is dissolved with 23mL acid solution to obtain solution A; the acid solution is hydrochloric acid solution; the concentration of the acid solution is 0.1mol / L;
[0032] Step 2, dissolving 60g of sulfobutyl-β-cyclodextrin in 400mL of water for injection to obtain solution B;
[0033] Step 3: Add solution A to solution B at a rate of 0.05 mL / s under stirring at room temperature to obtain a mixed system; the room temperature is 20°C to 25°C;
[0034] Step 4. Stir the mixed system described in Step 3 for 3 hours at room temperature to obtain a voriconazole solution clathrated with sulfobutyl-β-cyclodextrin; the room temperature is 20°C ...
Embodiment 2
[0042] This embodiment provides a preparation method of eye drops, the osmotic pressure of the eye drops is 280mOsm / L, the eye drops include voriconazole solution complexed with sulfobutyl-β-cyclodextrin, the drops The content of voriconazole in eye drops is 1mg / mL;
[0043] Described preparation method comprises:
[0044] Step 1, 1g voriconazole is dissolved with 8mL acid solution to obtain solution A; the acid solution is hydrochloric acid solution; the concentration of the acid solution is 1mol / L;
[0045] Step 2. Dissolve 15g of sulfobutyl-β-cyclodextrin in 100mL of water for injection to obtain solution B;
[0046] Step 3. Under the condition of stirring at room temperature, solution A is added to solution B at a rate of 0.25mL / s to obtain a mixed system; the stirring is by ultrasonic stirring;
[0047] Step 4. Stir the mixed system described in Step 3 for 3.5 hours at room temperature to obtain a voriconazole solution clathrated with sulfobutyl-β-cyclodextrin;
[0048...
Embodiment 3
[0053] This embodiment provides a method for preparing eye drops, the osmotic pressure of the eye drops is 320mOsm / L, the eye drops include voriconazole solution complexed with sulfobutyl-β-cyclodextrin, the drops The content of voriconazole in eye drops is 5mg / mL;
[0054] Described preparation method comprises:
[0055] Step 1, 5g voriconazole is dissolved with 30mL acid solution to obtain solution A; the acid solution is hydrochloric acid solution; the concentration of the acid solution is 0.5mol / L;
[0056] Step 2. Dissolve 75g of sulfobutyl-β-cyclodextrin in 500mL of water for injection to obtain solution B;
[0057] Step 3. Under the condition of stirring at room temperature, solution A is added to solution B at a rate of 0.2mL / s to obtain a mixed system; the stirring is by ultrasonic stirring;
[0058] Step 4. Stir the mixed system described in Step 3 for 4 hours at room temperature to obtain a voriconazole solution clathrated with sulfobutyl-β-cyclodextrin;
[0059]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com