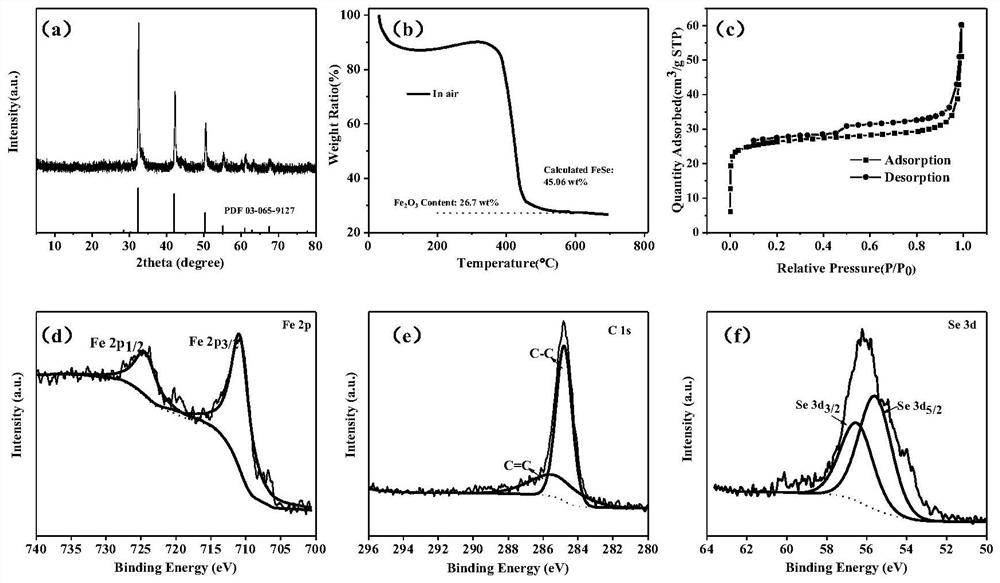
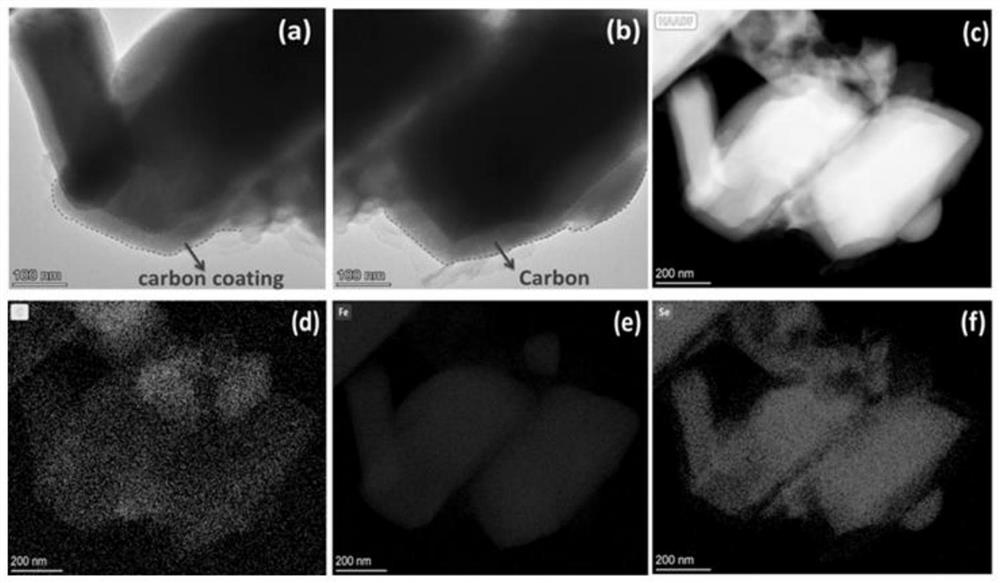
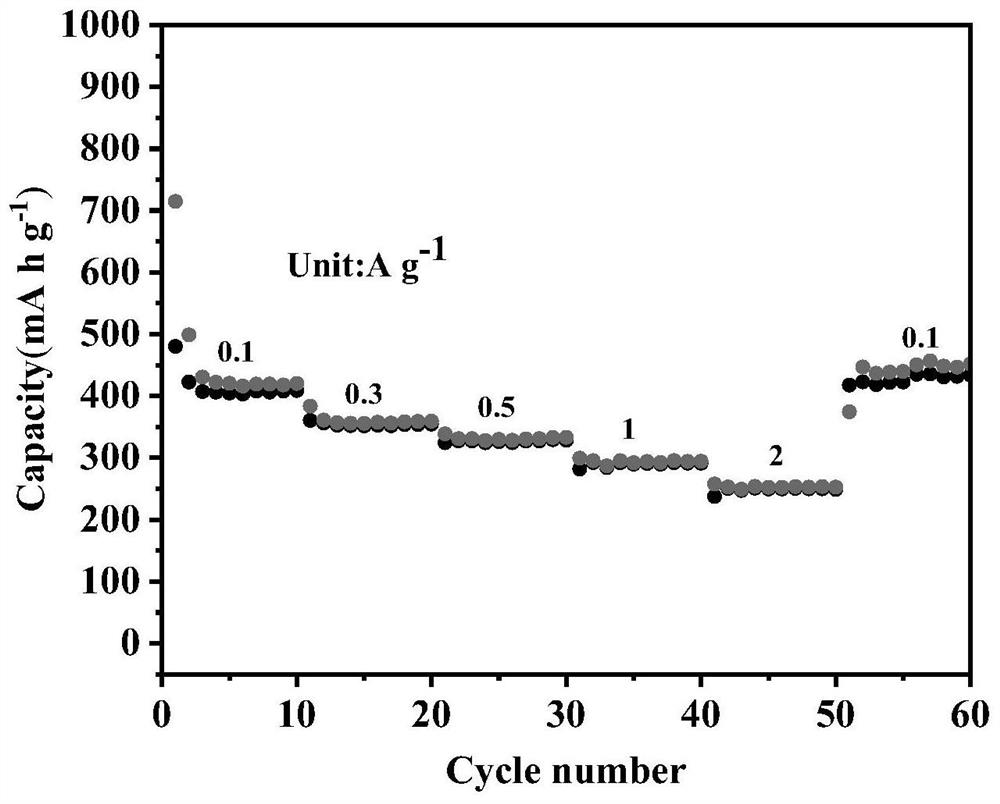
Porous iron selenide carbon-coated composite material and application thereof in potassium ion battery
A composite material, iron selenide technology, applied in porous iron selenide carbon-coated composite materials and its application in potassium ion batteries, can solve the problems of lithium resource consumption, lithium battery energy density can not be satisfied, etc., to achieve improvement Cycling performance, excellent cycle stability, effect of shortening the transfer path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 2g of ferric nitrate nonahydrate and 1.2g of benzenetricarboxylic acid in 25mL of deionized water respectively, after mixing, add 1mL of hydrofluoric acid, and stir for 30min. Transfer to a reaction kettle and keep at 150°C for 12h with a heating rate of 2°C / min. After cooling, wash and centrifuge. The sample was placed in a vacuum oven at 80°C for 12 hours, and after drying, the MIL-100(Fe) precursor was obtained.
[0027] Take 200 mg of MIL-100 (Fe) precursor and 300 mg of selenium powder, and use a ball mill at a speed of 200 r / min for 2 h for mixing. The mixture was placed in a tube furnace, and under the protection of an argon atmosphere, the temperature was raised at a rate of 5 °C / min, first kept at 260 °C for 5 h, and then kept at 720 °C for 4 h. After the reaction is finished, the black solid after the reaction is taken out to obtain a porous iron selenide carbon-coated composite material.
Embodiment 2
[0029] Dissolve 2g of ferric nitrate nonahydrate and 1.5g of benzenetricarboxylic acid in 25mL of deionized water respectively, after mixing, add 2mL of hydrofluoric acid, and stir for 30min. Transfer to a reaction kettle and keep at 160°C for 12h with a heating rate of 2°C / min. After cooling, wash and centrifuge. The sample was placed in a vacuum oven at 80°C for 12 hours, and after drying, the MIL-100(Fe) precursor was obtained.
[0030] Take 300 mg of MIL-100 (Fe) precursor and 300 mg of selenium powder, and use a ball mill at a speed of 400 r / min for 2 h for mixing. The mixture was placed in a tube furnace, and under the protection of an argon atmosphere, the temperature was raised at a rate of 5 °C / min, first kept at 280 °C for 3 h, and then kept at 720 °C for 4 h. After the reaction is finished, the black solid after the reaction is taken out to obtain a porous iron selenide carbon-coated composite material.
Embodiment 3
[0032] Dissolve 2g of ferric nitrate nonahydrate and 1.6g of benzenetricarboxylic acid in 25mL of deionized water respectively, after mixing, add 1mL of hydrofluoric acid, and stir for 30min. Transfer to a reaction kettle and keep at 170°C for 12h with a heating rate of 2°C / min. After cooling, wash and centrifuge. The sample was placed in a vacuum oven at 80°C for 12 hours, and after drying, the MIL-100(Fe) precursor was obtained.
[0033] Take 120 mg of MIL-100 (Fe) precursor and 200 mg of selenium powder, and use a ball mill at a speed of 200 r / min for 2 h for mixing. The mixture was placed in a tube furnace, and under the protection of an argon atmosphere, the temperature was raised at a rate of 5 °C / min, first kept at 260 °C for 2 h, and then kept at 720 °C for 4 h. After the reaction is finished, the black solid after the reaction is taken out to obtain a porous iron selenide carbon-coated composite material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com