Aflatoxin detoxification composition as well as preparation method and application thereof
A technology for aflatoxin and composition, applied in the field of aflatoxin detoxification composition and preparation thereof, can solve the problems of easy formation of other harmful substances, generation of harmful substances, and seldom application, etc., and achieves increasing daily weight gain and improving growth. Condition, effect of relieving symptoms of poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
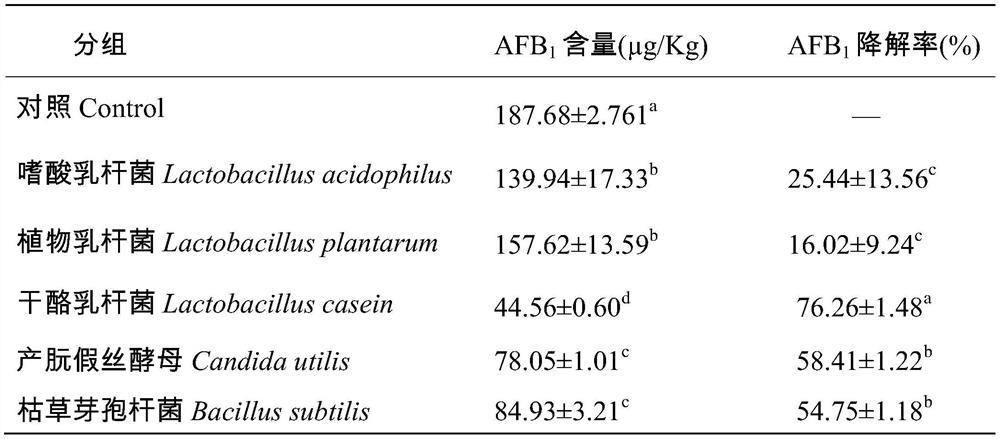
[0039]Embodiment 1, for degrading aflatoxin B 1 Screening of strains
[0040] 1. Activation of strains
[0041] Candida utilis preserved in the laboratory was inoculated into YPD medium, and cultured with shaking at 30°C and 180r / min for 24h. Bacillus subtilis was inoculated into LB liquid medium, and cultured with shaking at 37°C and 180r / min for 24h.
[0042] Lactobacillus (Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus plantarum) was inoculated into MRS liquid medium, and cultured at a constant temperature of 37° C. for 24 hours. After measuring the number of viable bacteria by plate coating method, the number of viable bacteria was uniformly adjusted to 1×10 9 Colonyformed unit (CFU) / mL, the number of live bacteria is expressed by natural logarithm value (lg), and the strains are stored in a refrigerator at 4°C for later use.
[0043] 1.2 Screening for degraded AFB 1 single bacteria
[0044] 1.2.1 Experimental design and experimental grouping
[0045]...
Embodiment 2
[0055] Embodiment 2, the application of detoxification composition
[0056] 1 Feeding experiment design and grouping
[0057] The experiment was carried out at the Xuchang Experimental Base of Henan Agricultural University. 200 healthy AA broilers were selected and randomly divided into 4 treatment groups, with 5 repetitions in each group and 10 chickens in each repetition. The feeding period is 21d. Adopt multi-layered cages, feed freely, light 24h in the first week, (after one week, 23h light, 1h dark); the control of temperature and humidity is carried out according to routine feeding and management process; Immunization program is: when broiler chicken 7d, with new - Immunization with a dual vaccine. The diet was formulated according to the NRC (1994) broiler feeding standard, and the feed formula and nutritional level are shown in Table 2. The test groups are as follows:
[0058] Group A: basal diet (positive control, AFB in the diet 1 3.04 μg / kg);
[0059] Group B...
Embodiment 3
[0105] Example 3, the composition for detoxification on broiler liver and jejunum structure and microbial flora
[0106] Test reagent: formalin solution.
[0107] 1 Collection of test materials
[0108] During the 21-day slaughter test, in 3 treatment groups (A, B, D), 6 chickens were selected for slaughter (half male and female) in each group, and the same part of the jejunum was taken about 1.5 cm, and the same part of the liver was taken with a weight of 5 g. Tissues were rinsed with saline and soaked in 10% formalin. The contents of the jejunum were placed in 2 mL sterile cryopreservation tubes and stored in liquid nitrogen.
[0109] 1.1 Tissue section making and microscope observation
[0110] After the samples were fixed, they were sent to the Department of Pathology, Henan Agricultural University for testing.
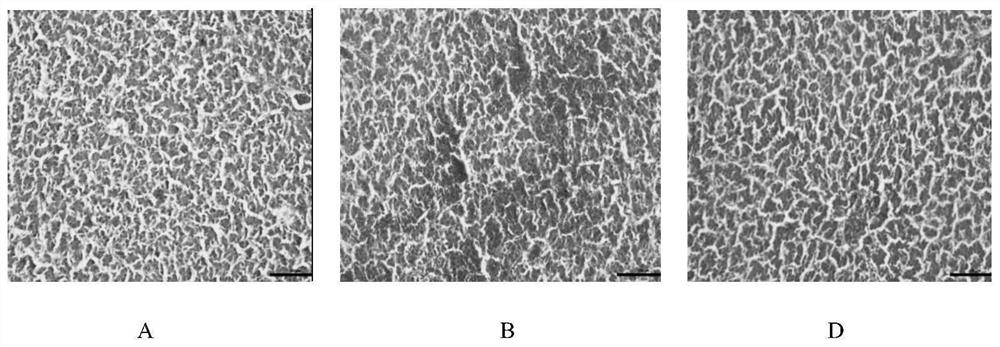
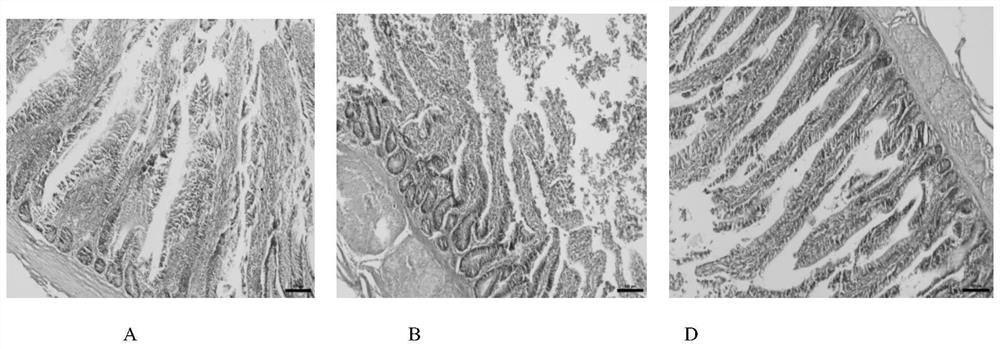
[0111] 2.1 Effects of different treatment groups on the tissue structure of broiler liver and jejunum
[0112] Tissue section of broiler liver see figure 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com