SKLB1039 compound, and preparation method and application thereof
A compound and reaction technology, applied in the field of preparation of new compounds, can solve problems such as poor physical and chemical properties, large amount of raw material reagents used, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
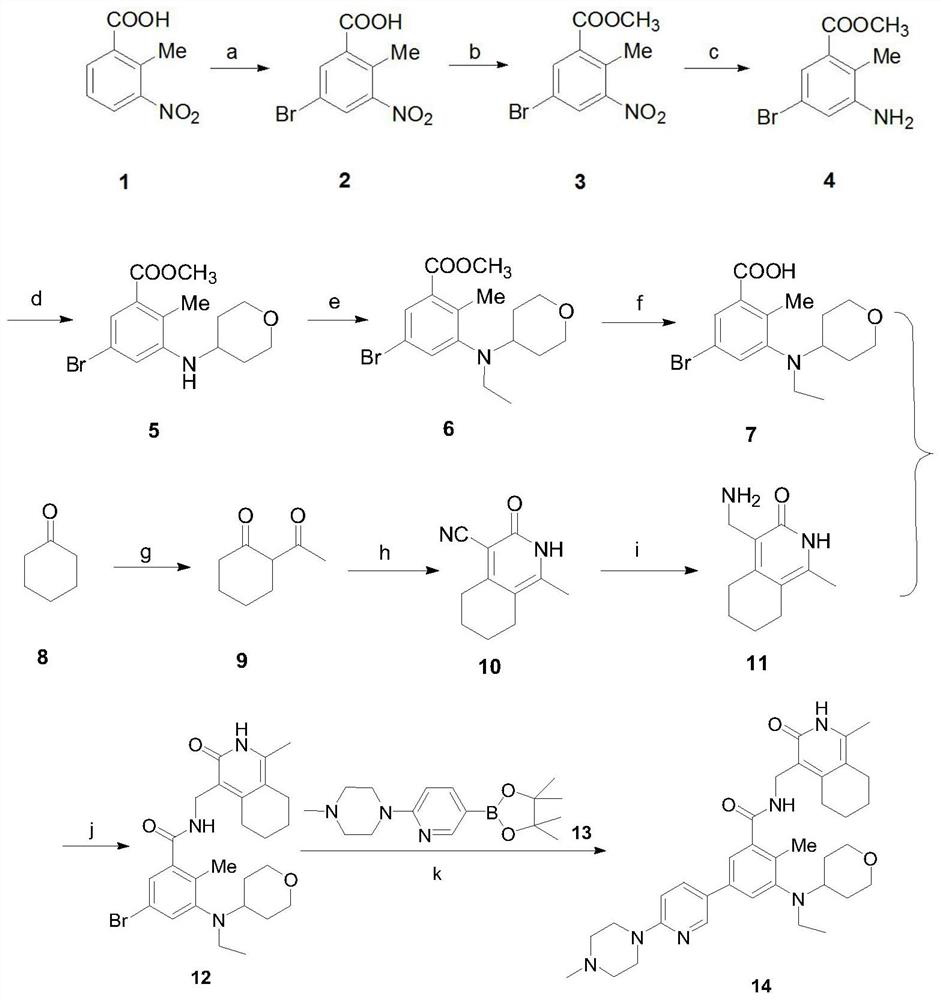
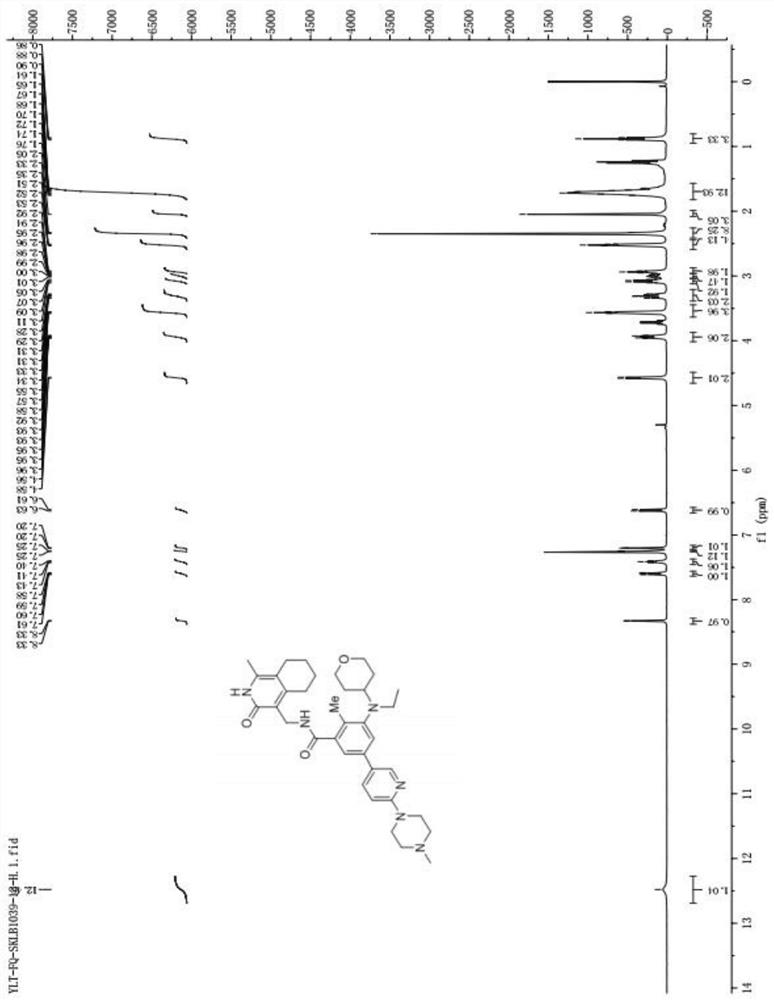
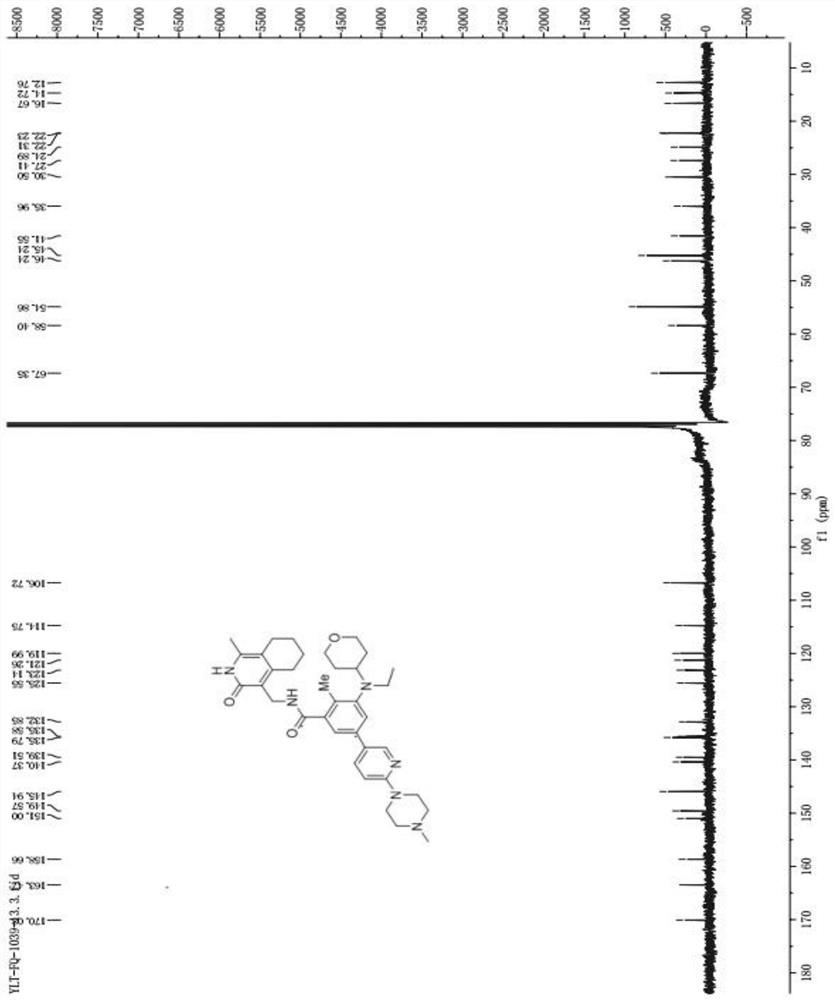
[0040] A preparation method of SKLB1039 compound, comprising the steps of:
[0041] Synthesis of S1, 5-bromo-2-methyl-3-nitrobenzoic acid (compound 2):
[0042] Under the protection of nitrogen, add 6.5L of concentrated sulfuric acid with a mass fraction of 98% in a 20L reactor, add 1.63kg of 2-methyl-3-nitrobenzoic acid (compound 1) under stirring, continue to stir and cool down to 3°C , until all the solids in the reactor are dissolved, and the solution is dark brown at this time; slowly add 1.425kg of 1,3-dibromo-5,5-dimethyl-2,4-imidazolidinedione in batches, during which the thermal effect Obviously, the temperature rises from 6°C to 19°C. After the addition, the solution in the reactor turns light brown and solids gradually precipitate out, making the system cloudy; keep the low temperature bath and cool it down to 10°C, then remove the low temperature bath. Warm bath, naturally heat up to 15-25°C and stir for 30 minutes, the solution in the reaction kettle gradually tu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com