Preparation method of S-cyanohydrin lyase and product thereof
A lyase and cyanohydrin technology, applied in the field of preparation of S-cyanohydrin lyase, can solve problems such as difficulty in meeting practical application requirements, insufficient enzyme activity, and low soluble protein content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The specific design principle of the present invention is to obtain various mutant cyanohydrin lyases by constructing random mutation and point saturation mutation libraries, and high-throughput screening.
[0029] The source of the S-cyanohydrin lyase mutant is wild-type cyanohydrin lyase, and its specific synthesis method is: the cyanohydrin lyase wild gene is shown in Seq ID No.1, or the cyanohydrin lyase mutant gene is shown in As shown in Seq ID No.2, restriction sites NdeI and HindIII were added at both ends, and the DNA was digested and purified and inserted into the expression vector pET26b(+) to obtain a recombinant plasmid, which was transformed into E.coliBL21(DE3) for construction into recombinant expression strains. The above recombinant strains were cultured in LB medium to OD 600 After = 1.0, 0.2mM IPTG was induced and cultured at 30°C for 4-5 hours, and the wild-type or mutant daughter cells were collected by centrifugation, and the crude enzyme solutio...
Embodiment 2
[0031] The preparation method of wild-type cyanohydrin lyase mutant is as follows:
[0032] The cyanohydrin lyase wild gene sequence of cassava (Manihot esculenta) is shown as Seq ID No.1, and the point of introduction is introduced by error-prone PCR and / or based on the enzyme protein structure / simulated substrate docking Saturation mutation followed by high-throughput screening to obtain the wild-type mutant of cyanohydrin lyase in our invention, as shown in Seq ID No.2.
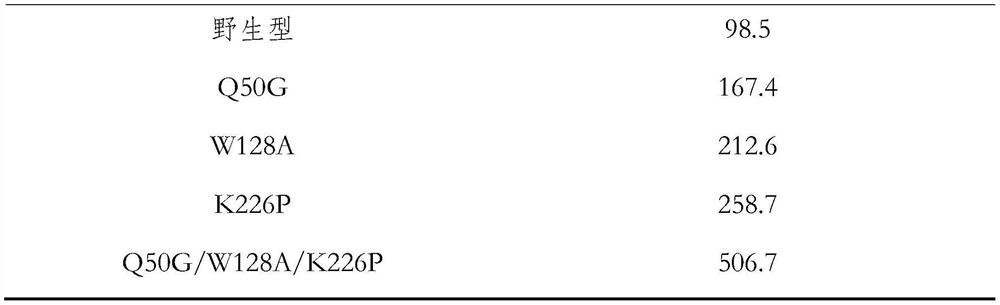
[0033] The protein sequence after transcription and translation of the cyanohydrin lyase wild gene of cassava (Manihot esculenta) is shown in Seq ID No.3, and the protein sequence translated by the wild-type cyanohydrin lyase mutant is shown in Seq ID No.4. Compared with the wild type cyanohydrin lyase mutant, glutamic acid at position 50 is mutated to glycine, tryptophan at position 128 is mutated to alanine, and lysine at position 226 is mutated to proline The difference, Seq ID No.4 represents the poss...
Embodiment 3
[0036] The steps for high-throughput screening of cyanohydrinase are as follows: at 25°C, add the following reagents in sequence in a 96-well plate, 130 μL of 100 mM potassium phosphate-citrate buffer (pH 5.0), 20 μL of diluted lyase solution, and finally add For 50 μL of phenytocyanine substrate solution, use a microplate reader to read the change in absorbance at 280 nm for 5 minutes, and the change in absorbance represents the level of enzyme activity.
[0037] Phenylethanol substrate solution: use 100 mM potassium phosphate-citrate buffer (pH 3.5) to prepare a concentration of 8 microliters per milliliter of buffer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com