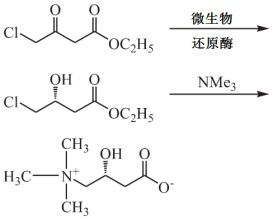
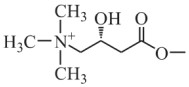
Preparation method of levocarnitine
A technology of coenzyme and reductase, applied in the field of drug synthesis, can solve problems such as poor stereoselectivity, and achieve the effects of improved yield, low environmental pollution and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The present embodiment provides a kind of preparation method of levocarnitine, comprises the following steps:
[0027] (1) Ethyl 4-chloroacetoacetate in methanol, ketoreductase KRED-101, coenzyme NAD + , glucose dehydrogenase, glucose and glucose solution, adjust the pH value to 7.0, stir at 25°C, and a reduction reaction occurs to generate (R)-4-chloro-3-hydroxybutyric acid ethyl ester; glucose solution The mass percent concentration of 4-chloroacetoacetate is 6.5%; the mass volume ratio of 4-chloroacetoacetate and first form is 1:8g / ml, and the mass volume ratio of 4-chloroacetoacetate and glucose solution is 1:40g / ml . The mass ratios of the carbonyl reductase, coenzyme, glucose dehydrogenase and ethyl 4-chloroacetoacetate are 0.65:1, 0.25:1 and 0.5:1 respectively;
[0028] (2) Ethyl (R)-4-chloro-3-hydroxybutyrate reacts with trimethylamine to generate levocarnitine, specifically: in the presence of sodium hydroxide, slowly add trimethylamine aqueous solution to (R...
Embodiment 2
[0030] The present embodiment provides a kind of preparation method of levocarnitine, comprises the following steps:
[0031] (1) Ethyl 4-chloroacetoacetate in ethanol, ketoreductase KRED-101, coenzyme NAD + , glucose dehydrogenase, glucose and glucose solution, adjust the pH value to 6.5, stir at 30°C, and a reduction reaction occurs to generate (R)-4-chloro-3-hydroxybutyrate ethyl ester; the quality of the glucose solution The percentage concentration is 5%; the mass volume ratio of ethyl 4-chloroacetoacetate to ethanol is 1:10g / ml, and the mass volume ratio of ethyl 4-chloroacetoacetate to glucose solution is 1:30g / ml. The mass ratios of the carbonyl reductase, coenzyme, glucose dehydrogenase and ethyl 4-chloroacetoacetate are 0.8:1, 0.2:1 and 0.6:1 respectively;
[0032] (2) Ethyl (R)-4-chloro-3-hydroxybutyrate reacts with trimethylamine to generate levocarnitine, specifically: in the presence of sodium hydroxide, slowly add trimethylamine aqueous solution to (R) -In the...
Embodiment 3
[0034] The present embodiment provides a kind of preparation method of levocarnitine, comprises the following steps:
[0035] (1) Ethyl 4-chloroacetoacetate in isopropanol, ketoreductase KRED-101, coenzyme NAD + , glucose dehydrogenase, glucose and glucose solution, adjust the pH value to 7.5, stir at 20°C, a reduction reaction occurs to generate (R)-4-chloro-3-hydroxybutyrate ethyl ester; the quality of glucose solution The percentage concentration is 8%; the mass volume ratio of ethyl 4-chloroacetoacetate to isopropanol is 1:5g / ml, and the mass volume ratio of ethyl 4-chloroacetoacetate to glucose solution is 1:50g / ml . The mass ratios of the carbonyl reductase, coenzyme, glucose dehydrogenase and ethyl 4-chloroacetoacetate are 0.5:1, 0.3:1 and 0.4:1 respectively;
[0036](2) Ethyl (R)-4-chloro-3-hydroxybutyrate reacts with trimethylamine to generate levocarnitine, specifically: in the presence of sodium hydroxide, slowly add trimethylamine aqueous solution to (R) -In the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com