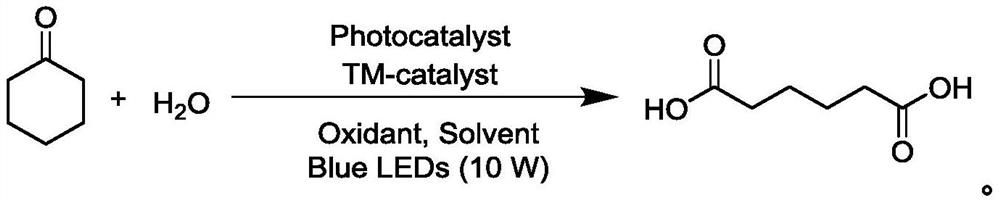
Preparation method of adipic acid
A technology of adipic acid and cyclohexanone, which is applied in the field of preparation of adipic acid, can solve the problems of high temperature, lowering, unfavorable production costs, etc., and achieve the effects of simple and gentle post-processing, increased dosage, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] This embodiment includes the following steps:
[0026] (1) In a 100mL Schlenk tube equipped with a magnet, add Cu(OTf) 2 (180mg, 5mol%), rosebic acid (145mg, 5mol%), cyclohexanone (980mg, 10mmol) were dissolved in 2mL of acetonitrile, added in the reaction tube under the air atmosphere, then deionized water (900mg, 50mmol ) was dissolved in 2 mL of acetonitrile, added to the reaction tube, and 46 mL of acetonitrile was added.
[0027] (2) The reaction was carried out under the irradiation of blue light (460-470nm, 10W), and the reaction was monitored through a TLC plate.
[0028] (3) Distill the reacted mixed solution under reduced pressure, remove the solvent, use 200-300 mesh silica gel for column chromatography separation, use ethyl acetate / petroleum ether (1:1) as eluent, or pass acetic acid Recrystallization from ethyl ester / n-hexane gave adipic acid (0.89 g, yield 61%) as a white solid.
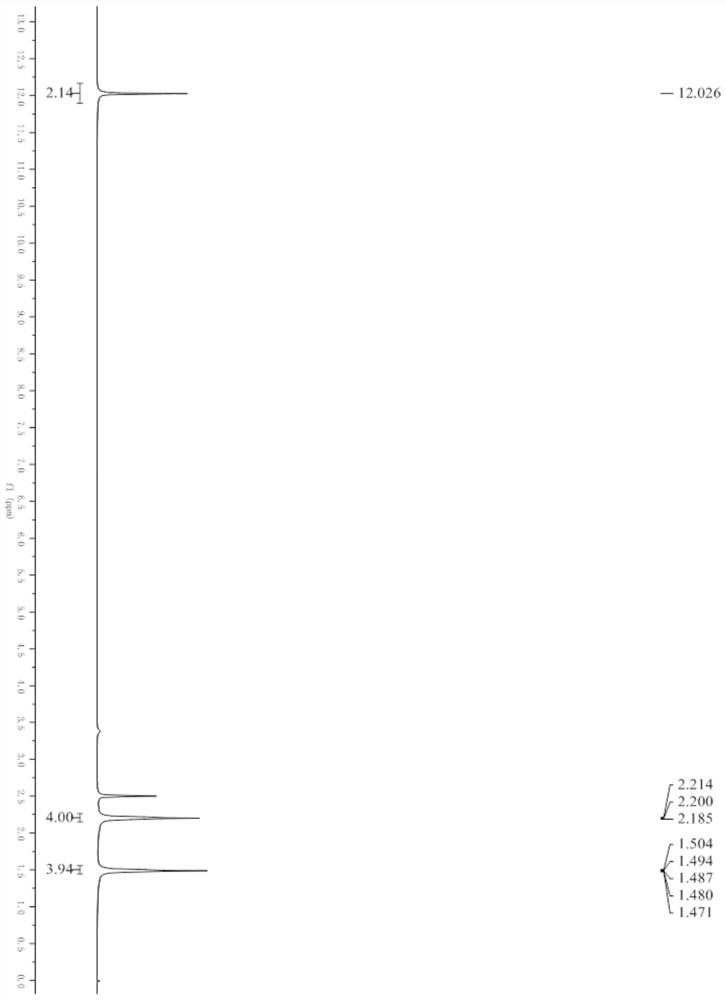
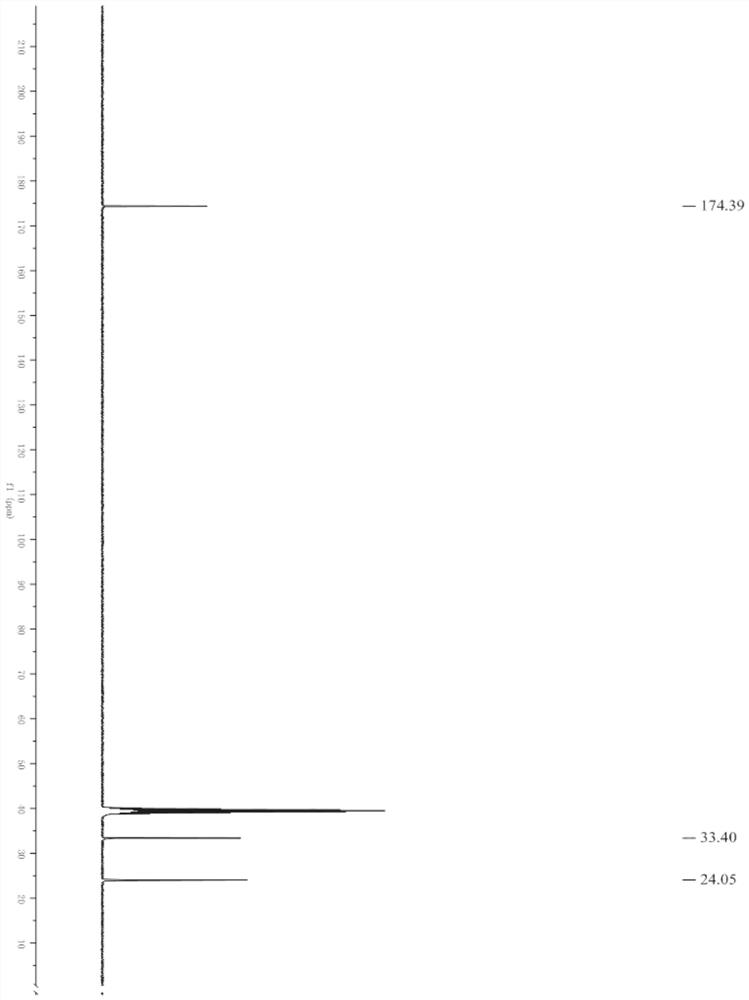
[0029] figure 1 , figure 2 Respectively the proton nuclear magnetic sp...
Embodiment 2
[0031] This embodiment includes the following steps:
[0032] (1) In a 100mL Schlenk tube equipped with a magnet, add Cu(OTf) 2 (180mg, 5mol%), rosebic acid (145mg, 5mol%); Oxygen was pumped out of the reaction tube three times by an oil pump, under an oxygen atmosphere, cyclohexanone (980mg, 10mmol) was dissolved in 2mL of acetonitrile, added to In the reaction tube, deionized water (900 mg, 50 mmol) was dissolved in 2 mL of acetonitrile, added to the reaction tube, and 46 mL of acetonitrile was added.
[0033] (2) The reaction was carried out under the irradiation of blue light (460-470nm, 10W), and the reaction was monitored through a TLC plate.
[0034] (3) Distill the reacted mixed solution under reduced pressure, remove the solvent, use 200-300 mesh silica gel for column chromatography separation, use ethyl acetate / petroleum ether (1:1) as eluent, or pass acetic acid Recrystallization from ethyl ester / n-hexane gave adipic acid (0.96 g, yield 66%) as a white solid.
Embodiment 3
[0036] This embodiment includes the following steps:
[0037] (1) In a 20mL Schlenk tube equipped with a magnet, add Cu(OTf) 2 (18mg, 5mol%), rosebic acid (14.5mg, 5mol%); the reaction tube was evacuated nitrogen three times through an oil pump, under nitrogen atmosphere, cyclohexanone (98mg, 1mmol) was dissolved in 1mL of acetonitrile, added In the reaction tube, dissolve deionized water (90 mg, 5 mmol) in 1 mL of acetonitrile and add to the reaction tube, dissolve hydrogen peroxide (113 mg, 2 mmol, 60%) in 1 mL of acetonitrile and add to the reaction tube , add 7 mL of acetonitrile.
[0038] (2) The reaction was carried out under the irradiation of blue light (460-470nm, 10W), and the reaction was monitored through a TLC plate.
[0039] (3) Distill the reacted mixed solution under reduced pressure, remove the solvent, use 200-300 mesh silica gel for column chromatography separation, use ethyl acetate / petroleum ether (1:1) as eluent, or pass acetic acid Ethyl ester / n-hexan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com