Medicine used for tumor treatment
A tumor treatment and drug technology, applied in the direction of anti-tumor drugs, drug combination, drug delivery, etc., can solve the problems of high incidence of side effects, large side effects, easy to produce drug resistance, etc., and achieve the effect of tumor suppression and metastasis inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of ORFV120 gene deletion strain:
[0051] The ORFV120 gene deletion strain of the present invention is prepared in the following manner:
[0052] (1) Prepare the parental strain: the parental strain is the ORFV-SY17 strain of the ovine infectious impetigo virus, and the full-length gene sequence of the ORFV-SY17 strain of the ovine infectious impetigo virus is shown in GenBank: MG712417.1 ;
[0053] (2) Construction of the screening expression cassette: inserting the specific promoter and the first marker gene suitable for the sheep infectious impetigo virus into the vector backbone to obtain the screening expression cassette;
[0054] (3) Construction of the shuttle plasmid: amplify the sequences on both sides of the ORFV120 gene deletion sequence of the ORFV-SY17 strain as the recombination homology arm, clone it into the screening expression cassette constructed in step (2), and obtain the marked recombination shuttle plasmid;
[0055] (4) using the shu...
Embodiment 2
[0074] Example 2 Detection of anti-tumor effect of ORFV120 gene deletion strain
[0075] In Example 2, the anti-tumor effect of the ORFV120 gene deletion strain obtained in Example 1 was tested.
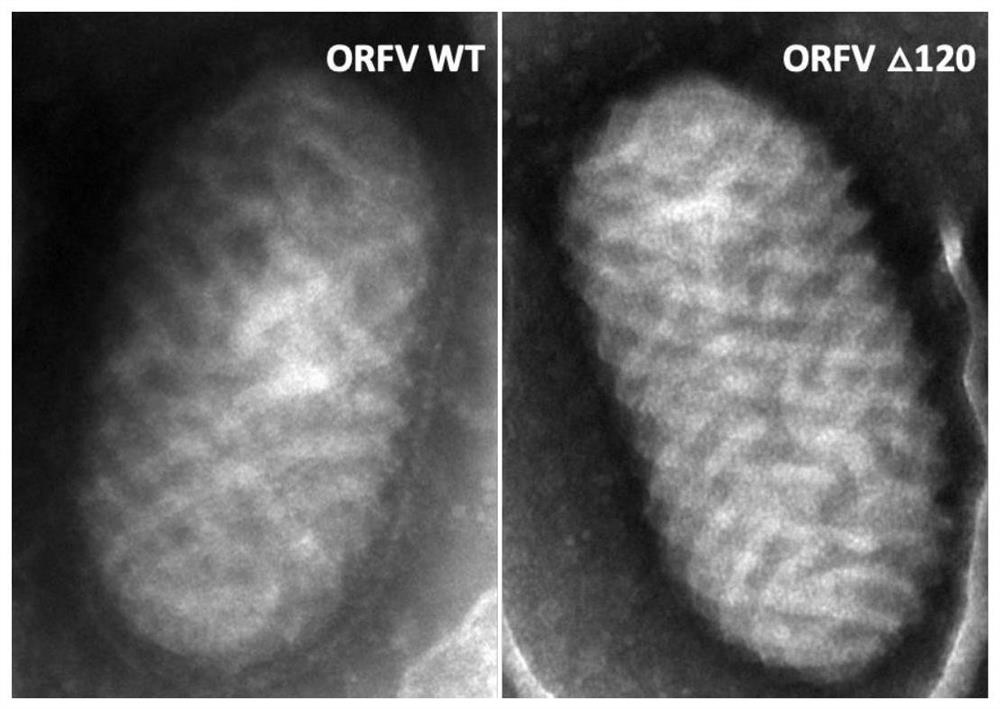
[0076] 1. Transmission electron microscope observation and identification of ORFV120 gene deletion strain
[0077] The ORFV120 gene deletion strain was observed and identified under a transmission electron microscope. The result is as figure 1 show.
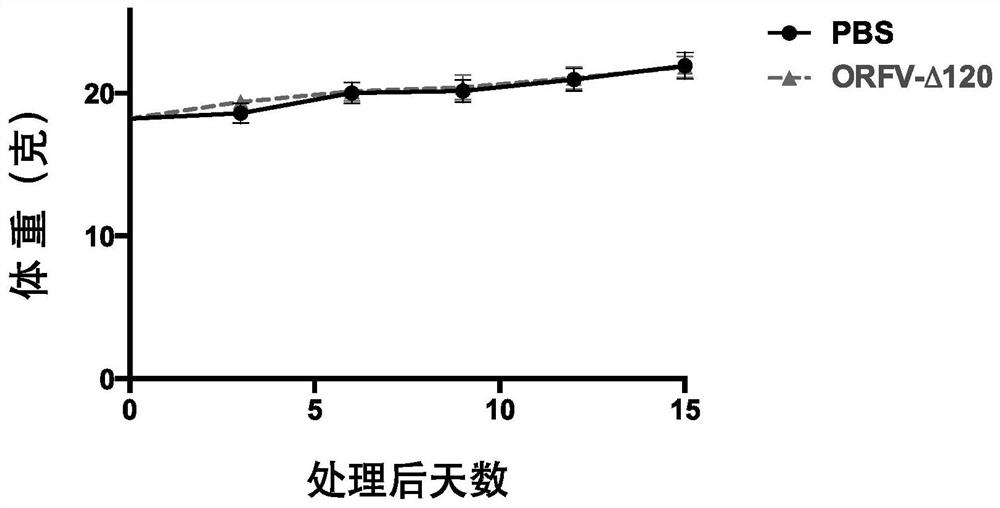
[0078] 2. Toxicity safety evaluation of ORFV120 gene deletion strain
[0079] During the implementation of the present invention, the ORFV120 gene deletion strain was intramuscularly injected into mice, and the injection dose was 2×10 6 TCID 50 , Record the body weight change of mice injected 0-15 days. The result is as figure 2 It was shown that the above viruses did not significantly change the body weight of mice.
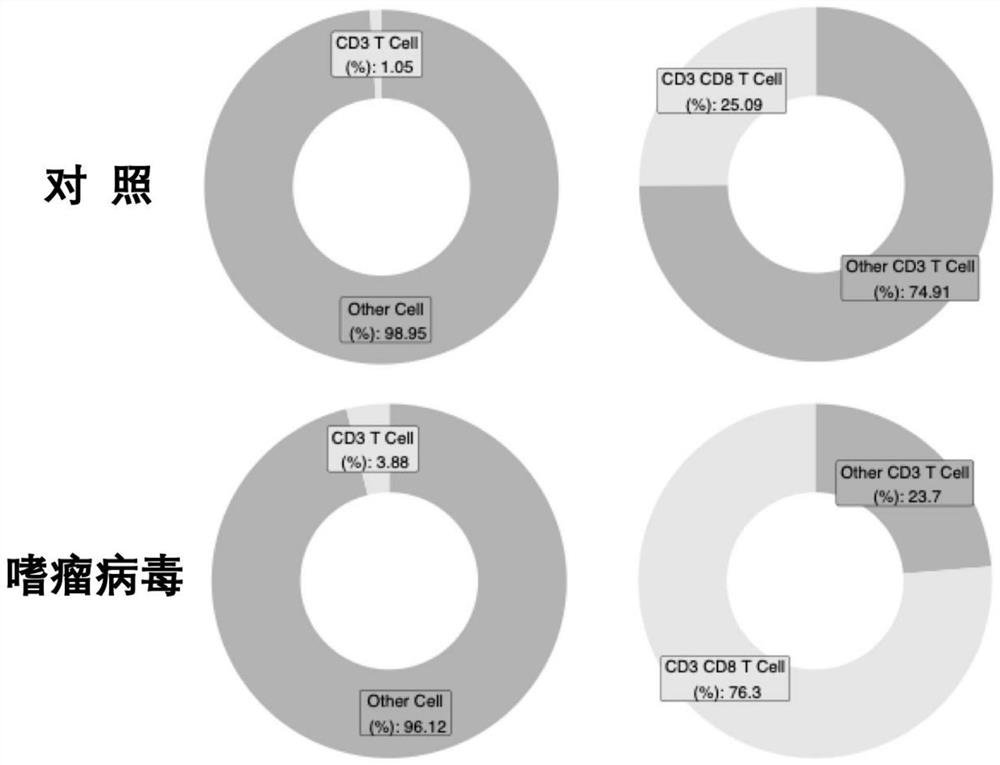
[0080] 3. The effect of therapeutic injection of ORFV120 gene deletion strain on the recruitment of immune cel...
Embodiment 3
[0089] Example 3 The anti-tumor drug obtained in Example 1 is used in combination with at least one of anti-tumor radiotherapy, anti-tumor chemotherapy, anti-tumor immunity, anti-tumor targeting, and anti-tumor hormone drugs. The combined chemical, antibody drugs, targeted inhibitors and hormone drugs are administered by intraperitoneal injection or oral administration, and the antitumor drugs including the attenuated strain of sheep infectious impetigo virus of the present invention are administered at staggered time intervals.
[0090] Combined use with anti-tumor chemotherapy: intraperitoneal injection of Etoposide (etoposide is a cell cycle-specific anti-tumor drug with a molecular formula of C 29 h 32 o 13 ) 10-50mg / kg, once every two days; the drug for tumor treatment including the attenuated strain of sheep infectious impetigo virus takes the form of intratumoral injection, staggered with anti-tumor chemotherapeutic drugs, and the dose is 10 6 TCID 50 .
[0091] Com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com