Catalyst for preparing butanol through ethanol coupling as well as preparation method and application of catalyst
A technology for preparing butanol and catalyst by ethanol coupling, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc. The effect of high conversion and excellent catalytic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
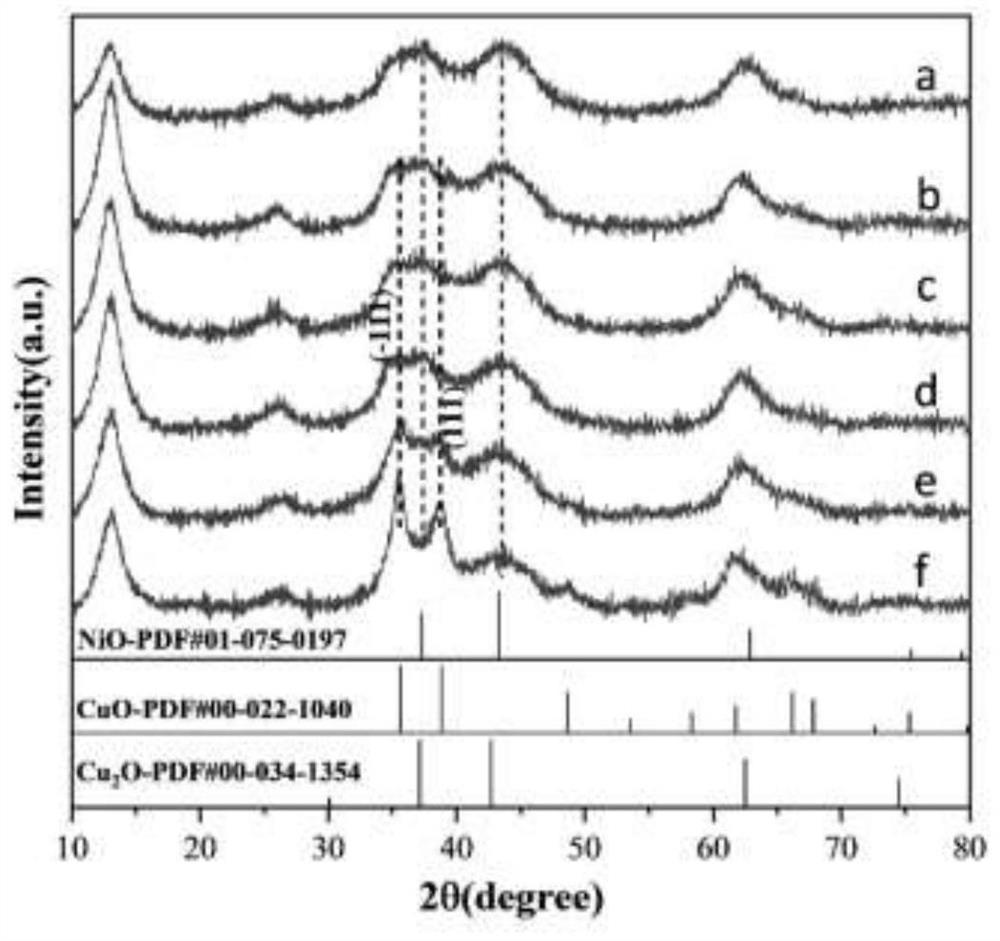
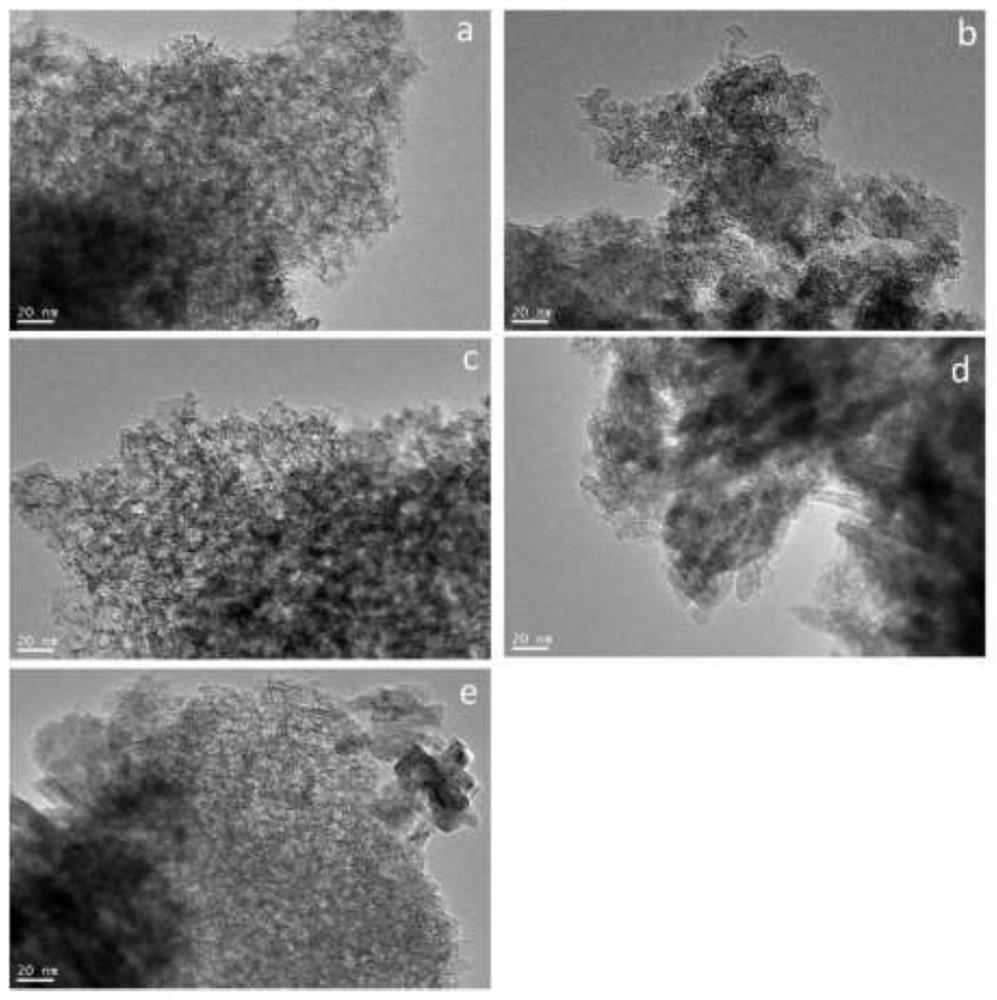
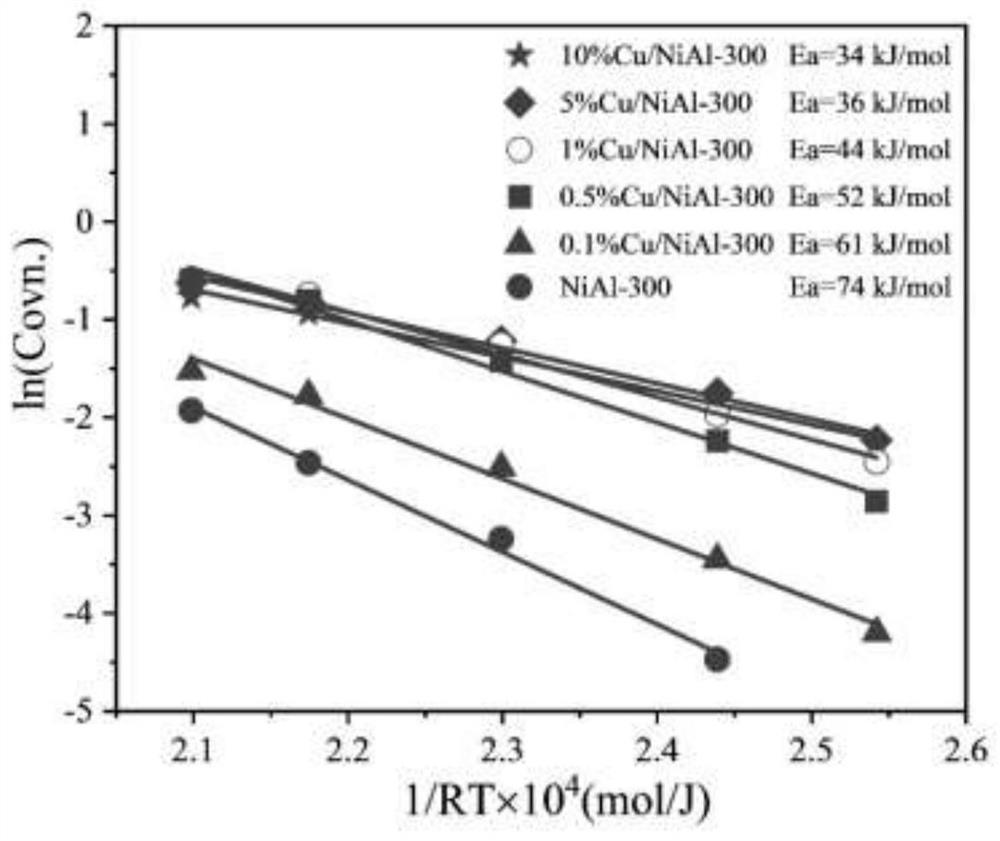
Image
Examples
Embodiment 1
[0034] Preparation of Supported Catalysts by Co-precipitation
[0035] Take Ni(NO 3 ) 2 ·6H 2 O 61.1g, Al(NO 3 ) 3 9H 2 O 26.2g (Ni / Al molar ratio is 3:1), Cu(NO 3 ) 2 ·6H 2 Add 2.07g of O into a 1000mL beaker, then add 200mL of deionized water and stir to dissolve to form solution A. Take NaOH0.438mol, NaOH 2 CO 3 Add 0.113mol into another beaker, add 200mL deionized water and stir to dissolve to form solution B. Under stirring in a water bath at 70°C, solution A was added dropwise into solution B at a rate of 5 mL / min with a constant flow pump, and 1M Na 2 CO 3 The pH value of the solution was adjusted to about 10, and then aged at this pH and temperature for 24 hours. After aging, the obtained suspension was repeatedly suction-filtered and washed with a large amount of deionized water until the pH value of the solution was 7, and then the filter cake was dried at 80° C. for 12 hours. Then it was raised from room temperature to 300°C at 5°C / min in air, and fir...
Embodiment 2
[0037] Preparation of Supported Catalysts by Deposition and Precipitation
[0038] Take Ni(NO 3 ) 2 ·6H 2 O 61.1g, Al(NO 3 ) 3 9H 2 Add 26.2g of O (Ni / Al molar ratio is 3:1) into a 1000mL beaker, then add 200mL of deionized water and stir to dissolve to form solution A. Take NaOH 0.438mol, Na 2 CO 3 Add 0.113mol into another beaker, add 200mL deionized water and stir to dissolve to form solution B. Under stirring in a water bath at 75°C, solution A was added dropwise into solution B at a rate of 5 mL / min with a constant flow pump, and 1M Na 2 CO 3 The pH value of the solution was adjusted to about 10, and then aged at this pH and temperature for 20 h. After aging, the obtained suspension was repeatedly suction-filtered and washed with a large amount of deionized water until the pH value of the solution was 7, and then the filter cake was dried at 120° C. for 8 hours to obtain a nickel-aluminum precursor. 0.2gCu(NO 3 ) 2 ·3H 2 The O solid was dissolved in 100 mL ...
Embodiment 3
[0040] Preparation of Supported Catalysts with Different Molar Ratio of Ni and Al by Co-precipitation
[0041] The difference from Example 1 is that Ni(NO 3 ) 2 ·6H 2 The amount of O was 40.7g, and the other steps and reagent amounts were the same as in Example 1, so the Ni / Al molar ratio in the catalyst thus obtained was 2:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com