Preparation process of benzyl glycolate
A technology for preparing benzyl glycolate and its preparation process, which is applied in the fields of carboxylate preparation, organic compound preparation, organic chemistry, etc., can solve the problems of unfavorable cost control, large amount of cesium carbonate, and high price, and is suitable for mass production , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
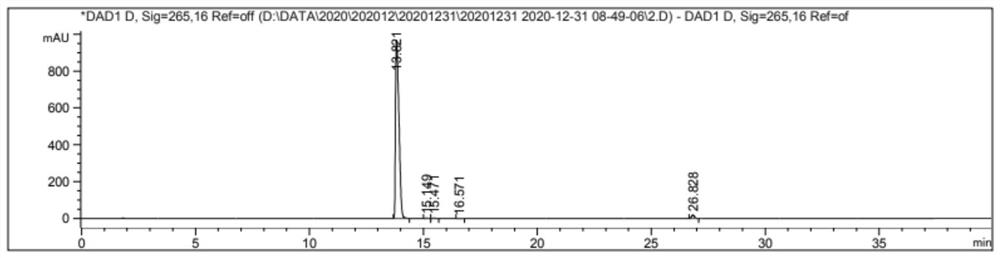
Embodiment 1
[0030]
[0031] Add glycolic acid (10 g, 0.13 mol) and 50 ml of absolute ethanol successively into a three-necked flask, and stir until completely dissolved.
[0032] Benzyl bromide (22.23 g, 0.13 mol) was added slowly, and the temperature of the reaction was controlled to 20°C, and stirred until completely dissolved.
[0033] A solution of sodium hydroxide (5.20 g, 0.13 mol) in absolute ethanol (10 ml) was added dropwise, and the temperature of the system was kept at 20° C. until the addition was complete.
[0034] The reaction was stirred for 10 h at room temperature.
[0035] Distill under reduced pressure until the volume of the reaction solution was reduced by half.
[0036] Add ethyl acetate (50ml*3) to extract the reaction solution, combine the organic phases, dry the extract with saturated sodium bicarbonate solution (300ml*4) and anhydrous magnesium sulfate successively, and evaporate the solvent to obtain a colorless liquid Benzyl glycolate Esters 17.69 g.
[0...
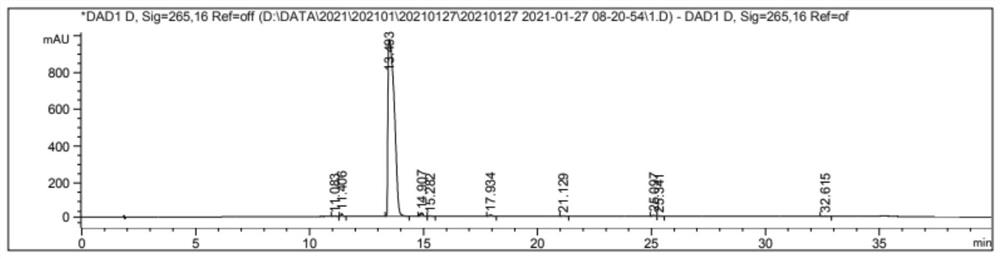
Embodiment 2
[0039]
[0040] Add glycolic acid (10 g, 0.13 mol) and 10 ml of acetone successively into a three-necked flask, and stir until completely dissolved.
[0041] Add benzyl bromide (22.23g, 0.13mol) slowly, and control the reaction temperature to -10°C, stir until completely dissolved.
[0042] A solution of triethylamine (13.20 g, 0.13 mol) in acetone (10 ml) was added dropwise, keeping the temperature of the system at -10°C until the addition was complete.
[0043] The reaction was stirred for 15 h at room temperature.
[0044] Distill under reduced pressure until the volume of the reaction solution is reduced to one-third.
[0045] Add ethyl acetate (50ml*3) to extract the reaction solution, combine the organic phases, dry the extract with saturated sodium bicarbonate solution (300ml*4) and anhydrous magnesium sulfate in turn, and evaporate the solvent to obtain dark yellow liquid Benzyl glycolate Esters 15.11 g.
[0046] The product that this embodiment obtains is dark i...
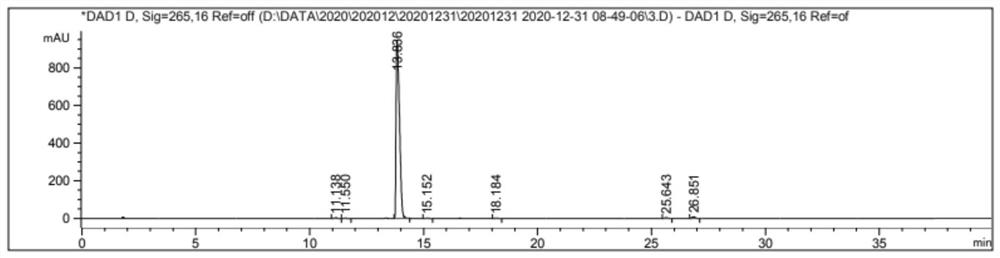
Embodiment 3
[0048]
[0049] Add glycolic acid (10 g, 0.13 mol) and 70 ml of acetonitrile successively into a three-necked flask, and stir until completely dissolved.
[0050] Benzyl bromide (21.58 g, 0.13 mol) was added slowly, and the reaction temperature was controlled to 0°C, and stirred until completely dissolved.
[0051] A solution of DBU (13.20 g, 0.13 mol) in acetonitrile (10 ml) was added dropwise, and the temperature of the system was kept at 0° C. until the addition was completed.
[0052] The reaction was stirred for 18h at room temperature.
[0053] Distill under reduced pressure until the volume of the reaction solution is reduced to one-fifth.
[0054] Add ethyl acetate (50ml*3) to extract the reaction solution, combine the organic phases, dry the extract with saturated sodium bicarbonate solution (300ml*4) and anhydrous magnesium sulfate successively, and evaporate the solvent to obtain a colorless liquid Benzyl glycolate Esters 19.85g.
[0055] The product purity th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com