Preparation method of NK1 receptor antagonist
A receptor antagonist, volume technology, applied in the field of preparation of NK1 receptor antagonist, can solve the problems of complex operation, low yield, long reaction time, etc., to shorten the reaction time, improve the crystallization efficiency and product yield and high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
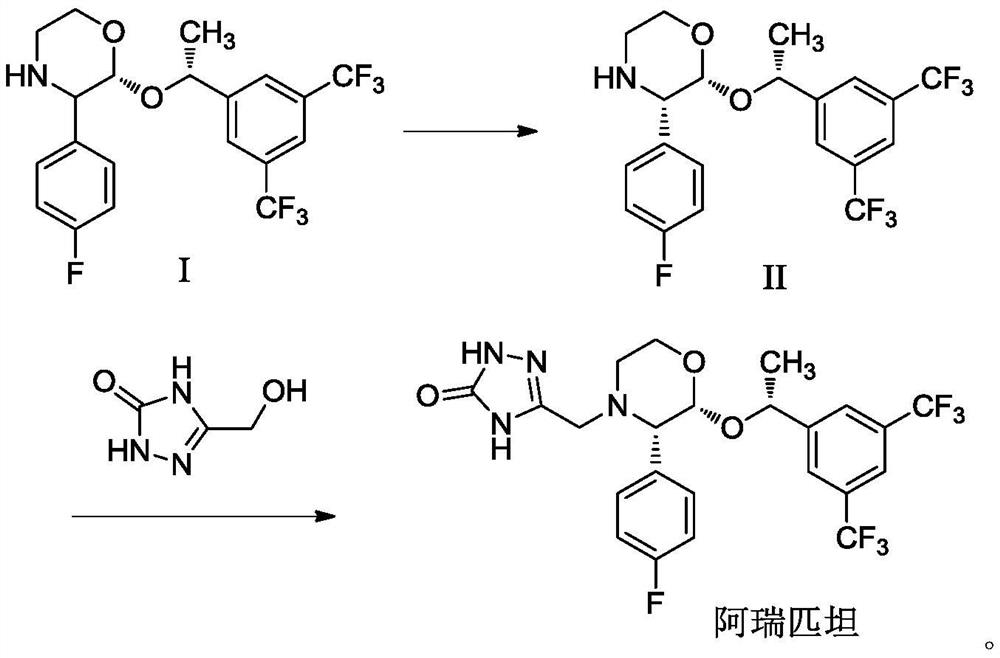
[0029] The synthesis of embodiment 1 aprepitant
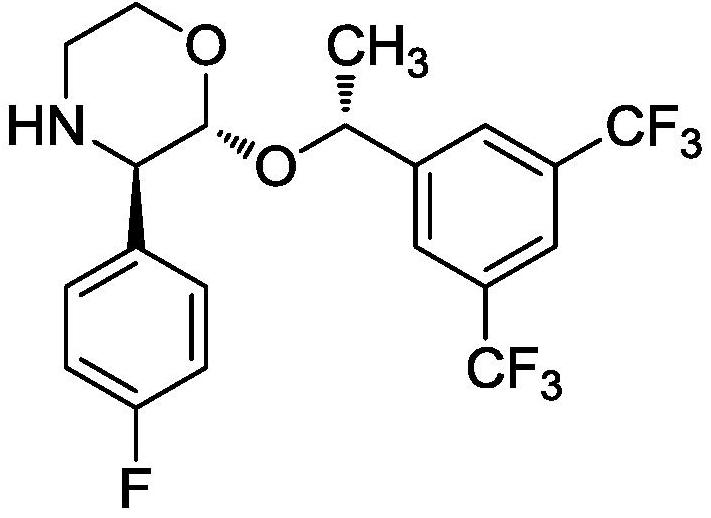
[0030] Dissolve 43.74g of compound I in 175mL of acetone-methyl tertiary ether (87.5mL+87.5mL) mixed solvent, add 20.62g of D-(-)-diethyl tartrate, add 2.59 g of N,N-diisopropylethylamine g, add CaCl 2 4.37g, react at room temperature for 4 hours, after the reaction, add 44mL of purified water, stir for 30min, filter, concentrate under reduced pressure to oil, add 175mL of purified water, stir at room temperature for 2h, filter and dry to obtain 40.33g of compound II, yield 92.2 %, the ee value is 99.8%.
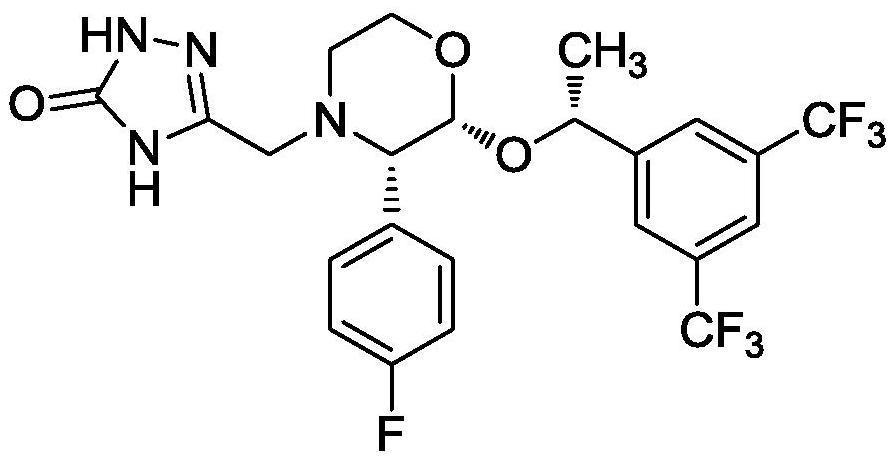
[0031] Dissolve 43.74g of compound II, 12.66g of 5-hydroxymethyl-2,4-dihydro-[1,2,4]triazol-3-one in 175mL ethyl acetate-acetone-methanol (105mL+35mL+ 35mL) mixed solvent, add 3.04g of 1,8-diazabicyclo[5,4,0-7-undecene], heat up to reflux, react for 2h, add anhydrous sodium sulfate and stir for 15min, filter, 40℃ Distilled under reduced pressure, added 260 mL of purified water, stirred and crystallized at room temperature,...
Embodiment 2
[0032] The synthesis of embodiment 2 aprepitant
[0033] Dissolve 43.74g of compound I in 220mL of acetone-methyl tertiary ether (73mL+147mL) mixed solvent, add 41.24g of D-(-)-diethyl tartrate, add 3.88g of N,N-diisopropylethylamine, Add CaCl 2 4.37g, react at room temperature for 4 hours, after the reaction, add 44mL of purified water, stir for 30min, filter, concentrate under reduced pressure to oil, add 262mL of purified water, stir at room temperature for 2h, filter and dry to obtain 39.76g of compound II, yield 90.9 %, the ee value is 99.6%.
[0034] Dissolve 43.74g of compound II, 12.66g of 5-hydroxymethyl-2,4-dihydro-[1,2,4]triazol-3-one in 220mL ethyl acetate-acetone-methanol (132mL+44mL+ 44mL) mixed solvent, add 4.57g of 1,8-diazabicyclo[5,4,0-7-undecene], heat up to reflux, react for 2h, add anhydrous sodium sulfate and stir for 15min, filter, 40℃ Distilled under reduced pressure, added 260 mL of purified water, stirred and crystallized at room temperature, filte...
Embodiment 3
[0035] The synthesis of embodiment 3 aprepitant
[0036] Dissolve 43.74g of compound I in 262mL of acetone-methyl tertiary ether (65.5mL+196.5mL) mixed solvent, add 20.62g of D-(-)-diethyl tartrate, add 5.17g of N,N-diisopropylethylamine g, add CaCl 2 4.37g, react at room temperature for 4 hours, after the reaction, add 44mL of purified water, stir for 30min, filter, concentrate under reduced pressure to oil, add 350mL of purified water, stir at room temperature for 2h, filter and dry to obtain 40.07g of compound II, yield 91.6 %, the ee value is 99.8%.
[0037] Dissolve 43.74g of compound II, 5-hydroxymethyl-2,4-dihydro-[1,2,4]triazol-3-one 12.66g in 262mL ethyl acetate-acetone-methanol (158mL+52mL+ 52mL) mixed solvent, add 6.09g of 1,8-diazabicyclo[5,4,0-7-undecene], heat up to reflux, react for 2h, add anhydrous sodium sulfate and stir for 15min, filter, 40℃ Distilled under reduced pressure, added 260 mL of purified water, stirred and crystallized at room temperature, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com