Antibodies against SARS-CoV-2 and uses thereof
A sars-cov-2 and antibody technology, applied in the direction of antibodies, applications, antiviral agents, etc., can solve problems such as limited clinical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
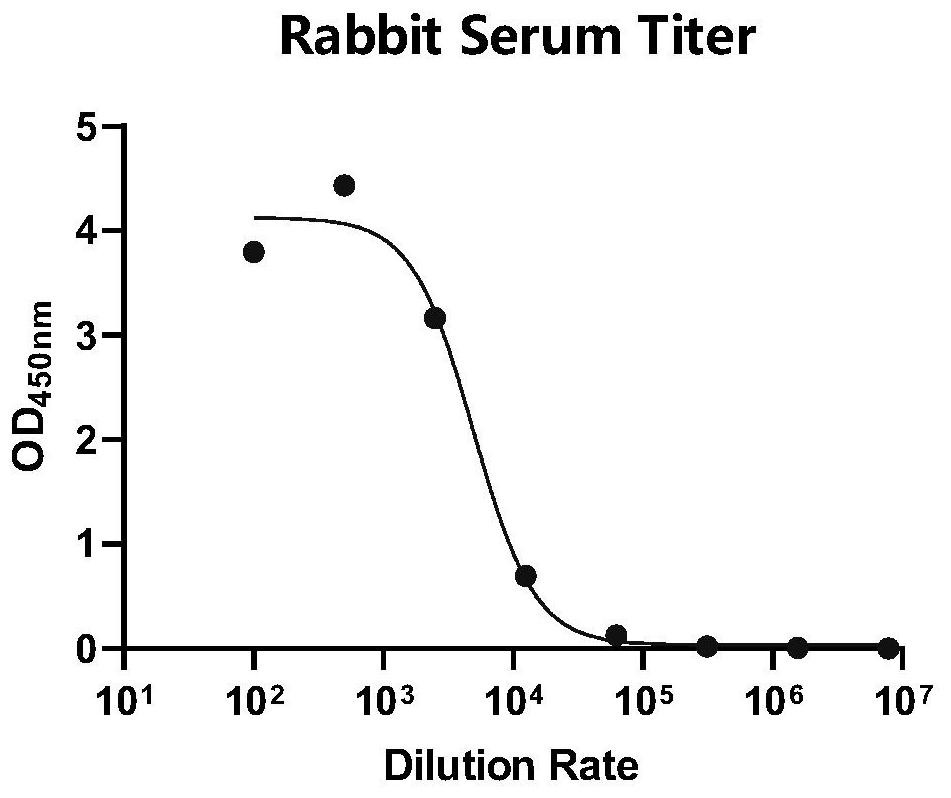
[0200] Example 1: Preparation of rabbit monoclonal antibody against SARS-CoV-2 receptor binding region RBD protein
[0201] 1.1 Preparation and activity identification of RBD protein in the receptor binding region of SARS-CoV-2:
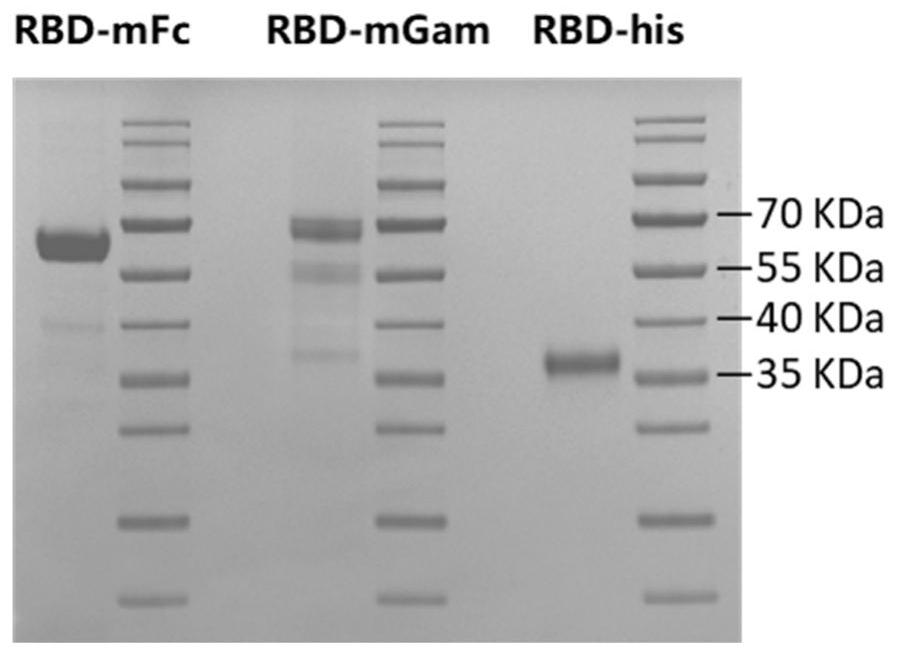
[0202] The SARS-CoV-2 receptor binding region RBD-mFc was purchased from Beijing Yiqiao Shenzhou Technology Co., Ltd., and the RBD gene of the SARS-CoV-2 receptor binding region RBD-His protein was obtained from the NCBI database (GenBank ID: MN908947.3 ), with a His tag fused at the C-terminus. Referring to the full gene sequence of SARS-CoV2-2 (MN908947.3), the full-length sequence of the outer region of the SARS-CoV2-2 spike protein (corresponding to the viral Spike gene aa316-aa550) amino acid sequence was optimized according to human preferred codons to obtain its optimized code nucleic acid sequence. The N-terminal of the RBD nucleic acid sequence is connected to the signal peptide coding sequence, the C-terminal is connected to the optimized...
Embodiment 2
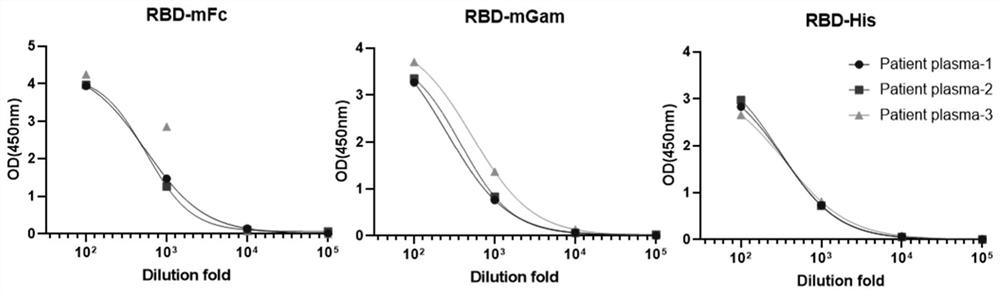
[0223] Example 2: Reactivity of anti-SARS-CoV-2 receptor binding domain protein RBD rabbit monoclonal antibody with SARS-CoV-2 receptor binding domain protein RBD
[0224] 2.1 Preparation of reaction plate
[0225] The SARS-CoV2-RBD (mFc tag) protein was treated with 50mM CB buffer (NaHCO 3 / Na 2 CO 3 Buffer, the final concentration is 50mM, the pH value is 9.6), and the final concentration is 2μg / mL; add 100μL of coating solution to each well of a 96-well microplate plate, and coat at 2~8℃ for 16~24 hours before Coat at 37°C for 2 hours; wash once with PBST washing solution (20mM PB7.4, 150mMNaCl, 0.1% Tween20); then add 200μL of blocking solution (pH containing 20% calf serum and 1% casein) to each well 20mM Na at 7.4 2 HPO 4 / NaH 2 PO 4 buffer solution), placed at 37°C for blocking for 2 hours; discard the blocking solution. After drying, put it into an aluminum foil bag and store it at 2-8°C for later use.
[0226] 2.2 ELISA detection of RBD rabbit monoclonal an...
Embodiment 3
[0229] Example 3: Anti-SARS-CoV-2 receptor binding region protein RBD rabbit monoclonal antibody and SARS-CoV-2 receptor binding region protein RBD affinity constant detection
[0230] Kinetic analysis of the binding of monoclonal antibody and antigen using Biacore 8K system, all steps are carried out under PBS buffer, using the company's matching Protein A chip to capture monoclonal antibody diluted to 5 μg / mL, using SARS-CoV -2 Receptor binding region protein RBD-His is used as the detection antigen, and the antigen is diluted into five gradients of 100nM, 50nM, 25nM, 12.5nM, and 6.75nM respectively during detection. The detection is carried out according to the following procedure: capture (Captue) for 60s, analyze ( Analyte) 300s, dissociation (Dissociation) 600s, regeneration (Regeneration) 60s. The data acquisition and analysis software supporting the instrument was used to calculate the affinity equilibrium dissociation constant.
[0231] The result is as Figure 6 Sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com