Novel alginate lyase BY17PV7 and application thereof
A kind of alginate lyase, a new type of technology, applied in the field of bioengineering, can solve the problems of low yield, cumbersome process of separating and extracting alginate lyase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
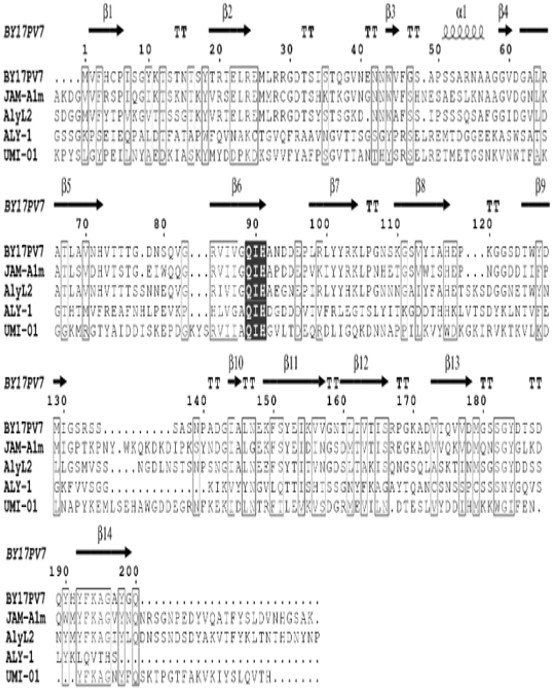
[0033] Example 1 Source and sequence analysis of alginate lyase BY17PV7
[0034] Microvesicles used in this example Microbulbifer sp. BY17 is provided by Fuzhou University.
[0035] The alginate lyase BY17PV7 of the present invention consists of by17pv7 The gene is expressed, its amino acid sequence is shown in SEQ ID No.1, and the nucleotide sequence of its coding gene is shown in SEQ ID No.2.
[0036] Amplified using V7 conserved primers Microbulbifer sp. The conserved region fragment of BY17 was amplified by SiteFingding PCR by17pv7 The nucleotide sequence of the gene is SEQ ID No.1, which is 681bp in length and encodes 227 amino acids. The sequence shown in SEQ ID No.1 is uploaded to NCBI to obtain the sequence number KY783478.1. The sequence of the V7 conservative primer is as follows:
[0037] V7F (SEQ ID No. 3): 5'-GTTGTTGTCGGNCARATHCAYGCN-3';
[0038] V7R (SEQ ID No. 4): 5'-TTGACCRTAAGCNCCNGCYTTRAARTA-3'.
[0039] The analysis of the conserved structure in NCB...
Embodiment 2
[0041]Example 2 Recombinant expression of alginate lyase BY17PV7
[0042] by Bam H I and xho I is the enzyme cutting site, the design primer makes the obtained in embodiment 1 by17pv7 Restriction sites are placed at both ends of the gene sequence.
[0043] Forward primer BY17PV7FB (SEQ ID No.5): 5'-GC GGATCC ATGGTATTCCACTGCCCGAT-3' (underlined as Bam H I restriction site);
[0044] Reverse primer BY17PV7RX (SEQ ID No.6): 5'-CC CTCGAG GTCGGCGTAGCCGGTGTGGCT-3’ (underlined xho I restriction site).
[0045] The PCR reaction was carried out according to the following conditions: pre-denaturation at 95°C for 5 min; denaturation at 95°C for 30 s; annealing at 50°C for 20 s; extension at 72°C for 60 s, for a total of 30 cycles, followed by extension at 72°C for 10 min. The PCR SuperMix used in the PCR reaction was purchased from Beijing Quanshijin Biotechnology Co., Ltd.
[0046] Restriction enzymes BamHI and xho I performed double enzyme digestion on the PCR produc...
Embodiment 3
[0048] Example 3 Induced fermentation of alginate lyase BY17PV7
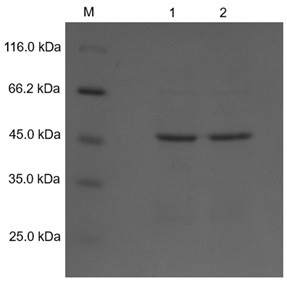
[0049] Resuscitate the recombinant alginate lyase-expressing bacteria BL21-pGEX-BY17PV7, transfer it to 1L LB liquid medium containing a final concentration of 50 ug / ml Amp+ according to the inoculation amount of 1% volume ratio, cultivate at 37°C for 4 hours, and then add The final concentration of 0.4 mM isopropyl thiogalactopyra Noside (isopropyl-β-D-galactopyraNoside, IPTG), induced at 20 ℃ for 18 h. Centrifuge at 10,000 rpm at 4°C for 8 min, resuspend the bacteria in pre-cooled distilled water, centrifuge at 10,000 rpm at 4°C for 15 min, resuspend in deionized water (repeat three times), and completely remove the culture medium. The precipitate was dissolved with buffer1 and ultrasonically broken for 35 min. Then centrifuge at 12,000 rpm and 4°C for 15 min, collect the supernatant, and obtain the crude enzyme solution.
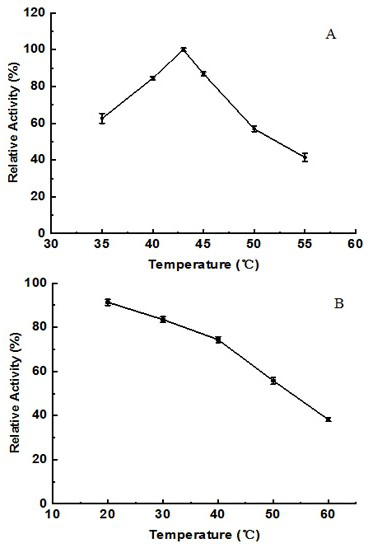
[0050] The enzymatic activity of alginate lyase was determined by measuring the amoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com