Process for preparing sodium carbonate crystal by wet pyrolysis of sodium bicarbonate solid
A technology of sodium bicarbonate solid and sodium carbonate, applied in carbonate preparations, alkali metal carbonates, chemical instruments and methods, etc., can solve problems such as long time and unreasonable economy, and achieve high decomposition rate, high concentration High and easy recycling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
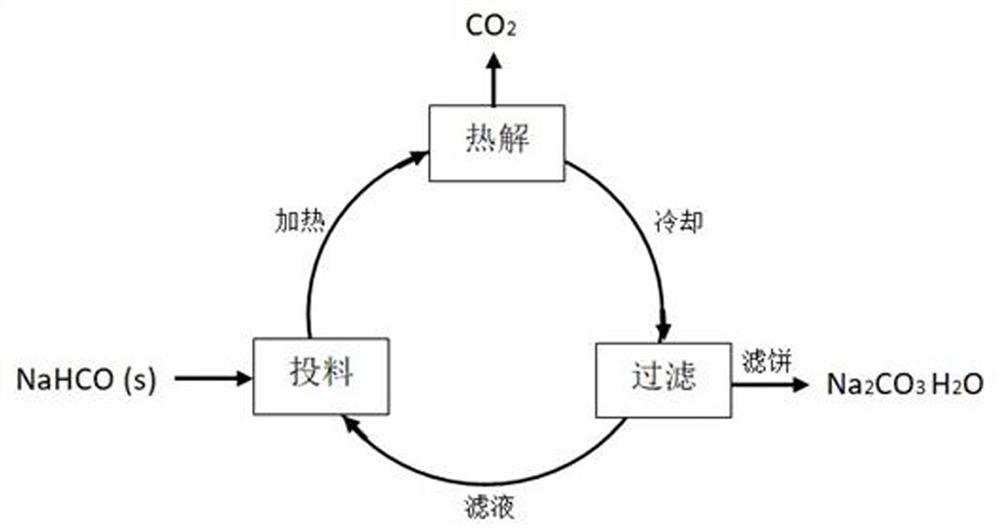
[0040] A kind of technology that sodium bicarbonate solid wet pyrolysis prepares sodium carbonate crystal, comprises the following steps:
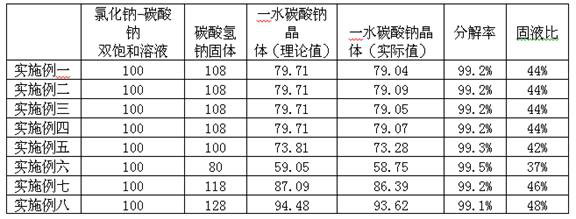
[0041] S1: In 100 parts by weight of sodium chloride-sodium carbonate double saturated solution, while stirring and heating, slowly add 108 parts by weight of sodium bicarbonate solid;
[0042] S2: After adding the material, continue to stir and heat up to 90 ° C, when no carbon dioxide gas is generated, stop heating;
[0043] S3: Cool and filter to obtain sodium carbonate monohydrate crystals.
[0044] In S1, the method for preparing sodium chloride-sodium carbonate double saturated solution: add excessive sodium chloride, sodium carbonate solid in water, stir to form double saturated solution, filter out unnecessary sodium chloride, sodium carbonate solid, filtrate is Sodium chloride-sodium carbonate double saturated solution.
[0045] Such as figure 1 Shown, in the preparation technology of the present invention, sodium chloride-sodi...
Embodiment 2
[0053] A kind of technology that sodium bicarbonate solid wet pyrolysis prepares sodium carbonate crystal, comprises the following steps:
[0054] S1: In 100 parts by weight of sodium chloride-sodium carbonate double saturated solution, while stirring and heating, slowly add 108 parts by weight of sodium bicarbonate solid;
[0055] S2: After adding the material, continue to stir and heat up to 118 ° C, when no carbon dioxide gas is generated, stop heating;
[0056] S3: Cool and filter to obtain sodium carbonate monohydrate crystals.
[0057] The preparation method of the sodium chloride-sodium carbonate double-saturated solution is the same as that of Example 1, and will not be repeated.
[0058] In the preparation process of the embodiment of the present invention, in S2, the temperature is raised to 118°C. Since the boiling point of brine is higher than that of water, and as the concentration of brine increases, the boiling point will also increase, and the sodium chloride-...
Embodiment 3
[0060] A kind of technology that sodium bicarbonate solid wet pyrolysis prepares sodium carbonate crystal, comprises the following steps:
[0061] S1: In 100 parts by weight of sodium chloride-sodium carbonate double saturated solution, while stirring and heating, slowly add 108 parts by weight of sodium bicarbonate solid;
[0062] S2: After adding the material, continue to stir and heat up to 100 ° C, when no carbon dioxide gas is generated, stop heating;
[0063] S3: Cool and filter to obtain sodium carbonate monohydrate crystals.
[0064] The preparation method of the sodium chloride-sodium carbonate double-saturated solution is the same as that of Example 1, and will not be repeated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com