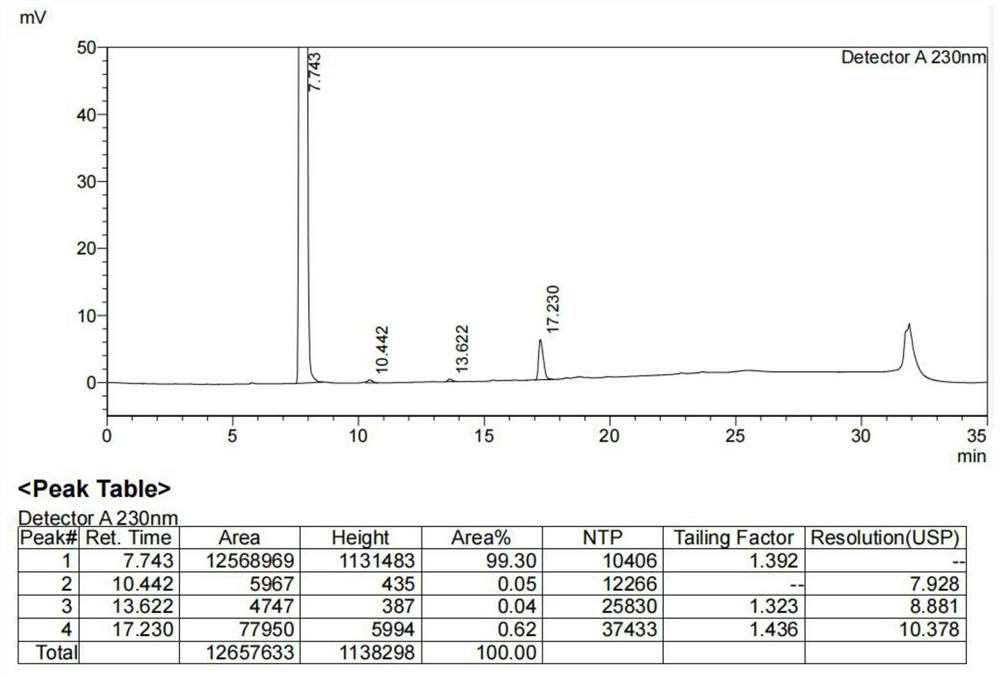
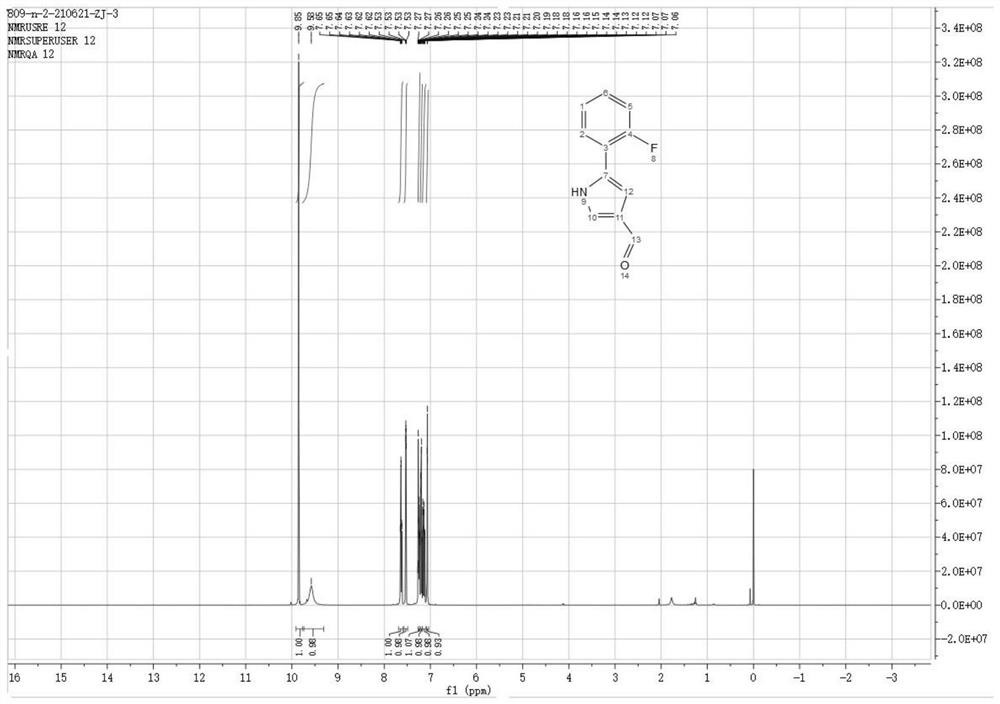
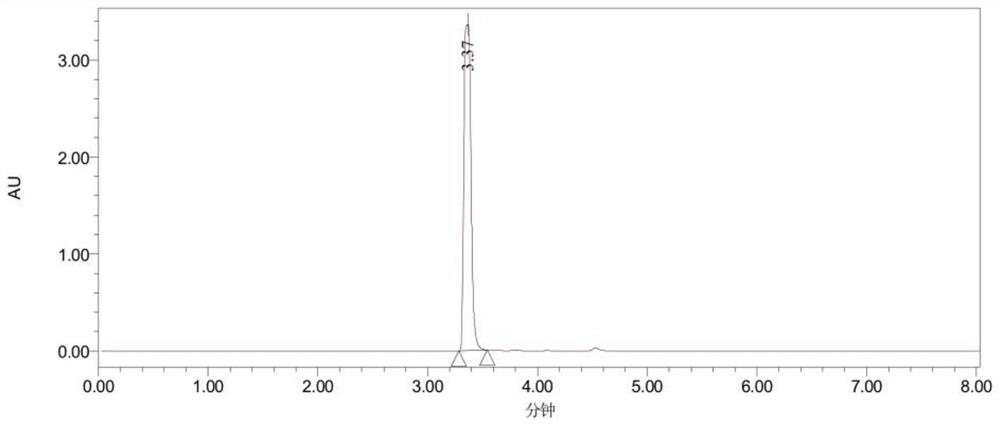
Preparation method of 5-(2-fluorophenyl)-1H-pyrrole-3-formaldehyde
A technology of fluorophenyl and pyrrole, applied in the field of preparation of 5--1H-pyrrole-3-carbaldehyde, can solve problems such as unfavorable industrialized production, long process route, unfavorable industrialization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The invention provides a kind of preparation method of 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde, comprising the following steps:
[0037] Mix 2-[2-(2-fluorophenyl)-2-oxoethyl]malononitrile, Raney nickel, solvent, acidic pH regulator and reducing agent, and carry out one-step reaction under acidic conditions to obtain 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde.
[0038] In the present invention, the 2-[2-(2-fluorophenyl)-2-oxoethyl]malononitrile is preferably a conventional commercially available product, more preferably purchased from Shandong Xuande Pharmaceutical Technology Co., Ltd._. In the present invention, the mass ratio of the 2-[2-(2-fluorophenyl)-2-oxoethyl]malononitrile and Raney nickel is preferably 1:0.2~0.5, more preferably 1: 0.35-0.49.
[0039] In the present invention, the solvent preferably includes one or more of alcohol, water and tetrahydrofuran, more preferably a mixture of alcohol and water or a mixture of tetrahydrofuran and water. In th...
Embodiment 1
[0073] 36.5g of 2-[2-(2-fluorophenyl)-2-oxoethyl]malononitrile purchased from Shandong Xuande Pharmaceutical Technology Co., Ltd., 130mL of tetrahydrofuran, 105mL of acetic acid and 50mL of water were stirred at 25°C Mix to obtain the first reaction solution;
[0074] Disperse 18g of Raney nickel in the first reaction solution to obtain a second reaction solution;
[0075] Add 120mL of sodium hypophosphite aqueous solution with a mass concentration of 0.76g / mL to the second reaction solution dropwise at a rate of 12 drops / min, and the addition is completed within 30 minutes; one-step reaction at 40°C for 3 hours;
[0076] Filter the product after the reaction, wash the filter residue twice with 50mL tetrahydrofuran, separate the filtrate obtained by filtration, and distill the upper organic layer at 60°C under reduced pressure; dissolve the solid obtained by distillation in 200mL ethyl acetate and 200mL molar concentration In the mixed solution of 1mol / L hydrochloric acid aqu...
Embodiment 2
[0079] 20.2g of 2-[2-(2-fluorophenyl)-2-oxoethyl]malononitrile purchased from Shandong Xuande Pharmaceutical Technology Co., Ltd., 80mL of ethanol, 60mL of acetic acid and 30mL of water were stirred at 25°C Mix to obtain the first reaction solution;
[0080] Disperse 10g of Raney nickel in the first reaction solution to obtain a second reaction solution;
[0081] Add 700mL of sodium hypophosphite aqueous solution with a mass concentration of 0.84g / mL to the second reaction solution dropwise at a rate of 13 drops / min, and the addition is completed within 30 minutes; one-step reaction at 38°C for 3 hours;
[0082] Filter the product after the reaction, wash the filter residue twice with 50mL tetrahydrofuran, separate the filtrate obtained by filtration, and distill the upper organic layer at 60°C under reduced pressure; dissolve the solid obtained by distillation in 200mL ethyl acetate and 200mL molar concentration In the mixed solution of hydrochloric acid aqueous solution of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com