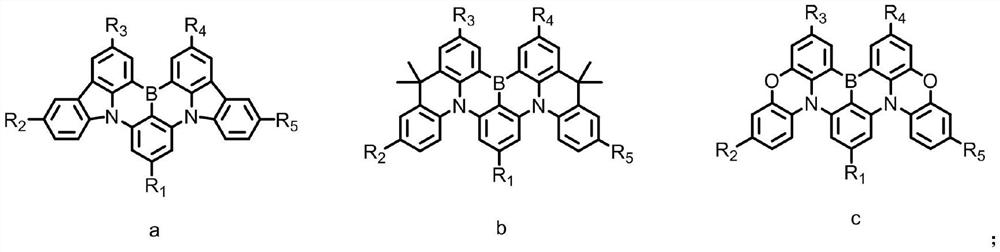
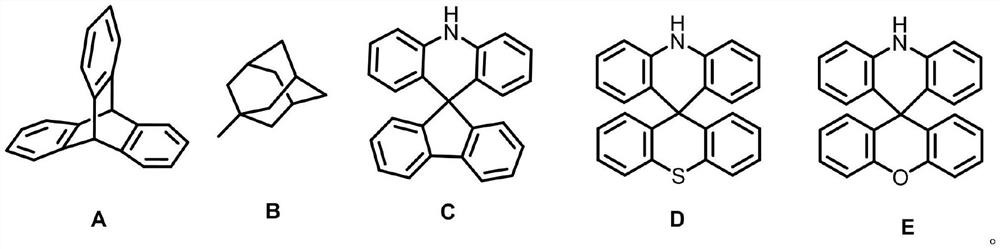
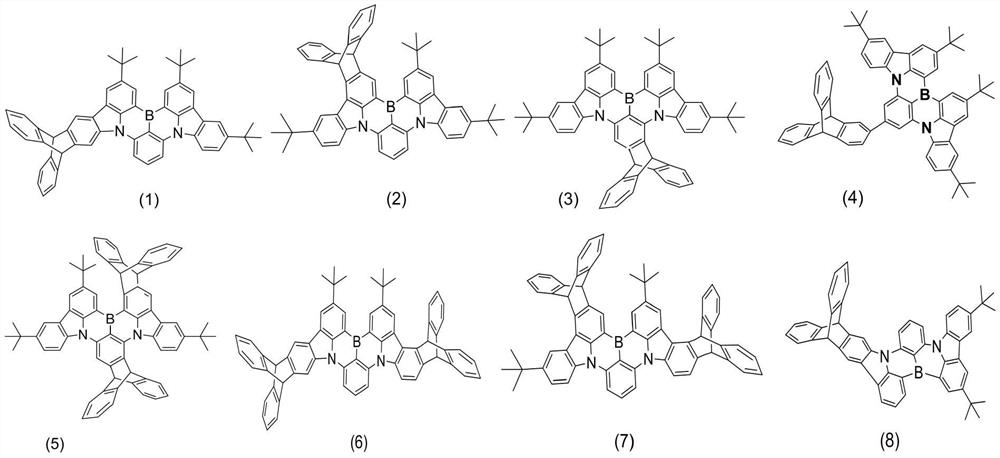
Multi-resonance type thermally activated delayed fluorescence material with spatial three-dimensional structure, electronic device and application of multi-resonance type thermally activated delayed fluorescence material
A thermal activation delay and spatial three-dimensional technology, applied in the direction of luminescent materials, electric solid-state devices, semiconductor devices, etc., can solve the problems of reducing the efficiency of OLED devices, and achieve the goals of improving solubility and thermal stability, good comprehensive performance, and increasing lifespan Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The specific synthesis process of the above-mentioned thermally activated delayed fluorescent material compound (4) is as follows:
[0039]
[0040] Preparation of Compound 2: Take a 100mL two-necked flask, add 2-bromotriptycene (1.67g, 5mmol), pinacol diboronate (1.30g, 5.1mmol), potassium acetate (0.98g, 10mmol) under the protection of argon ), Pd(dppf) 2 Cl 2 (0.36g, 0.5mmol) and 25mL of 1,4-dioxane, reacted at 100°C for 8 hours, cooled to room temperature, filtered through diatomaceous earth, and extracted three times with DCM (dichloromethane) and water . The combined organic phases were washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified by silica gel column chromatography using DCM / PE (v / v=1 / 4) as eluent to obtain 1.7 g of white solid with a yield of 90%.
[0041] 1 H NMR (500MHz, CDCl 3 )δ[ppm]: 7.26–7.31(m,4H),7.22–7.18(m,2H),7.13(d,J=8.2Hz,2H),7.10–7.08(m,2H),7.2(d,J =8.6Hz, 1H), 5.18(t, J=6.9H...
Embodiment 2
[0049] The specific synthesis process of the above-mentioned thermally activated delayed fluorescent material compound (8) is as follows:
[0050]
[0051] Preparation of compound 3: Take a 100mL two-necked flask, add compound 2 (2.26g, 5mmol), triptycene (1.75g, 5.1mmol), palladium acetate (0.05g, 0.25mmol), sodium tert-butoxide under the protection of argon (0.75g, 8mmol), tri-tert-butylphosphonium tetrafluoroborate (0.14g, 0.5mmol) and 25mL of anhydrous toluene were stirred and refluxed for 24 hours. After cooling to room temperature, it was filtered off through celite and extracted three times with DCM and water. The combined organic phases were washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified by silica gel column chromatography using DCM / PE (v / v=1 / 3) as eluent to obtain 2.44 g of white solid with a yield of 90%.
[0052] 1 H NMR (500MHz, CDCl 3 )δ[ppm]: 8.56–8.51(m,2H),7.92–7.88(m,4H),7.76(d,J=8.2Hz,1H),7....
Embodiment 3
[0057] The specific synthesis process of the above-mentioned thermally activated delayed fluorescent material compound (27) is as follows:
[0058]
[0059] The preparation of compound 3: get 100mL two-necked flask, add compound 2 (2.26g, 5mmol) under argon protection, compound 3 (2.23g, 5.1mmol), palladium acetate (0.05g, 0.25mmol), sodium tert-butoxide ( 0.75 g, 8 mmol), tri-tert-butylphosphonium tetrafluoroborate (0.14 g, 0.5 mmol) and 25 mL of anhydrous toluene were stirred and refluxed for 24 hours. After cooling to room temperature, it was filtered off through celite and extracted three times with DCM and water. The combined organic phases were washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified by silica gel column chromatography using DCM / PE (v / v=1 / 3) as eluent to obtain 3.09 g of white solid with a yield of 75%.
[0060] 1 H NMR (500MHz, CDCl 3 )δ[ppm]: 8.76–8.61(m,2H),7.76(d,J=8.2Hz,4H),7.65–7.54(m,2H),7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com