Ion rectification method for preparing lithium chloride from salt lake brine
A technology of salt lake brine and lithium chloride, which is applied in the field of ion distillation for the production of lithium chloride, can solve problems such as limiting the separation efficiency of target ions, unsatisfactory screening performance, and continuous operation characteristics that cannot be effectively reflected. Reduce the requirements for its own characteristics, simplify the construction method, and improve the effect of purity and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
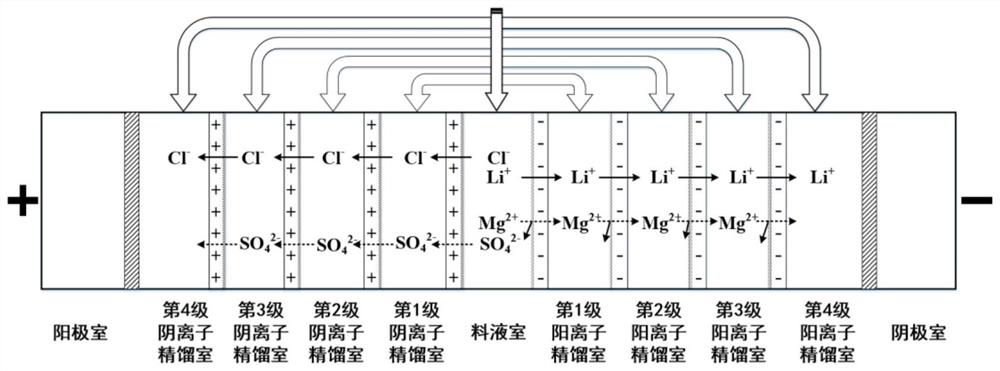
[0057] The present embodiment utilizes the above-mentioned ion rectification system to process the simulated high magnesium-lithium ratio salt lake brine, the main components of the simulated brine are lithium ions, magnesium ions, and chloride ions, and the adopted ion rectification system is a four-stage ion rectification system, The number of ion rectification units is 1; the first-stage anion rectification chamber is communicated with the first-stage cation rectification chamber, and the circulating flow is driven by a solution-driven pump; the second-stage anion rectification chamber is communicated with the second-stage cation rectification chamber, The circulation flow is driven by the solution-driven pump; the third-stage anion rectification chamber is communicated with the third-stage cation rectification chamber, and the circulation flow is driven by the solution-driven pump; the fourth-stage anion rectification chamber is communicated with the fourth-stage cation rect...
Embodiment 2
[0061] The present embodiment utilizes the above-mentioned ion rectification system to process the simulated salt lake brine. The main components of the simulated brine are lithium ions, magnesium ions, chloride ions, and sulfate ions. The ion rectification system used is a four-stage ion rectification system. The number of distillation units is 1. The first-stage anion rectification chamber is communicated with the first-stage cation rectification chamber, and the circulating flow is driven by the solution-driven pump; the second-stage anion rectification chamber is communicated with the second-stage cation rectification chamber, and the circulation flow is driven by the solution-driven pump; The third-stage anion rectification chamber is communicated with the third-stage cation rectification chamber, and the circulating flow is driven by the solution-driven pump; the fourth-stage anion rectification chamber is communicated with the fourth-stage cation rectification chamber, a...
Embodiment 3
[0065] In this embodiment, the above-mentioned ion distillation system is used to process the actual salt lake old brine. The main components of the salt lake brine are lithium ion, magnesium ion, calcium ion, chloride ion and sulfate ion, and the adopted ion rectification system is four-stage ion rectification. system, the number of ion distillation units is 1. The first-stage anion rectification chamber is communicated with the first-stage cation rectification chamber, and the circulating flow is driven by the solution-driven pump; the second-stage anion rectification chamber is communicated with the second-stage cation rectification chamber, and the circulation flow is driven by the solution-driven pump; The third-stage anion rectification chamber is communicated with the third-stage cation rectification chamber, and the circulating flow is driven by the solution-driven pump; the fourth-stage anion rectification chamber is communicated with the fourth-stage cation rectificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com