Preparation method of oseltamivir phosphate
A technology of oseltamivir phosphate and phosphoric acid, applied in the field of preparation of oseltamivir phosphate, can solve the problems such as the synthesis method of oseltamivir phosphate needs to be optimized and the purity of oseltamivir phosphate is low, and achieve high quality and purity , the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: a kind of preparation method of oseltamivir phosphate comprises the following steps:
[0042] S1, add 93g of trifluoroacetic acid and 45g of hydrochloride into the reaction flask, heat up to 48°C, react for 1h, concentrate under reduced pressure to recover trifluoroacetic acid, add 45g of toluene, cool down to 0°C, add 75g of distilled water, add 30% hydrogen Sodium oxide solution to adjust the pH to 12-13, add 172g of toluene, stand to separate layers, extract the organic phase with 40g of toluene, wash with 25% sodium chloride solution, separate the organic phase, dehydrate with anhydrous magnesium sulfate for 2h, and depressurize Concentrate, add 13g of ethyl acetate, stir at 38°C for 10min, add 129g of heptane, cool down to 25°C for crystallization, filter with suction, and dry in vacuum for 6h to obtain an intermediate product;
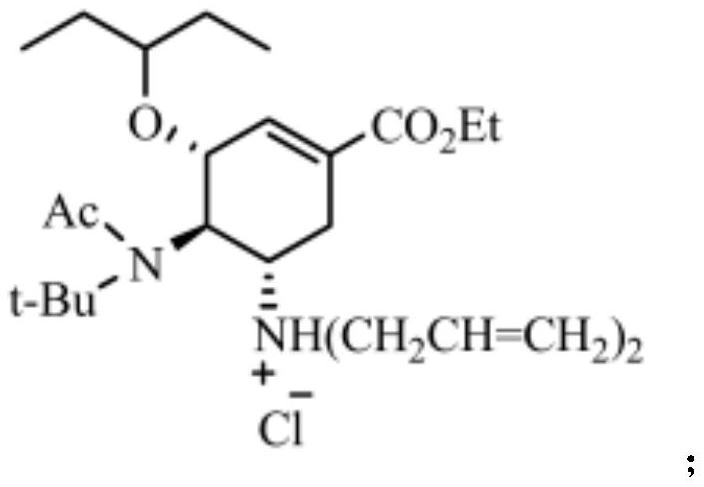
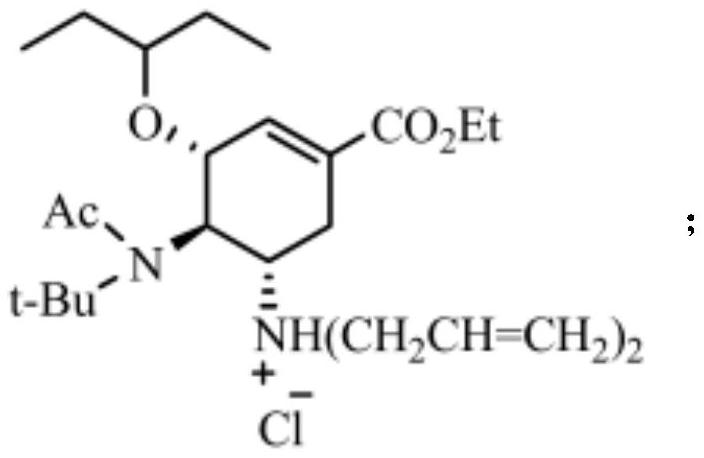
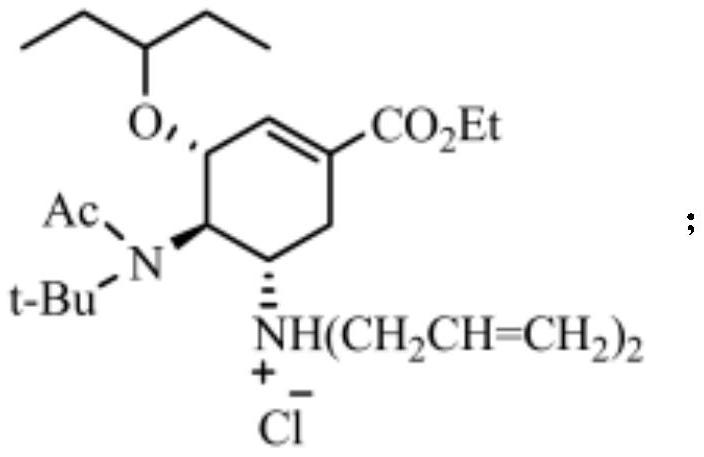
[0043] The structural formula of hydrochloride is:
[0044]
[0045] The structural formula of the intermediate product...
Embodiment 2
[0050] Embodiment 2: A kind of preparation method of oseltamivir phosphate, the difference with embodiment 1 is, in the preparation method of oseltamivir phosphate, the step of S1 is: mix 93g trifluoroacetic acid and 45g hydrochloride Add it into the reaction flask, heat up to 48°C, react for 1h, concentrate under reduced pressure to recover trifluoroacetic acid, add 45g of toluene, cool down to 0°C, add 75g of distilled water, add 30% sodium hydroxide solution to adjust the pH to 12-13, add 172g Toluene, standing for stratification, using 40g toluene to extract the organic phase for 3 times, combining the organic phase after extraction, washing with 25% sodium chloride solution, separating the organic phase, dehydrating with anhydrous magnesium sulfate for 2h, concentrating under reduced pressure, adding 13g of ethyl acetate was stirred at 38°C for 10min, 129g of heptane was added, the temperature was lowered to 25°C for crystallization, suction filtration, and vacuum drying f...
Embodiment 3
[0053] Embodiment 3: A preparation method of oseltamivir phosphate, the difference from Example 1 is that in S2 of the preparation method of oseltamivir phosphate, at a temperature of 45°C, first 50% of the filtrate Drop into phosphoric acid / ethanol solution, stir and react for 0.8h, then add dropwise the remaining filtrate, stir and react for 0.4h.
[0054] After analysis, the final product is the target product oseltamivir phosphate refined product, with a purity of 99.8%.
[0055] 1 H NMR (D 2 O, 400MHz) δ: 0.87 (t, J = 7.2Hz, 3H, CH 3 ), 0.91(t, J=7.2Hz, 3H, CH 3 ), 1.31(t, J=7.2Hz, 3H, CH 3 ), 1.47~1.52 (m, 1H, CH 2 ),1.56~1.62(m,3H,CH 2 ),2.11(s,3H,COCH 3 )2.51~2.58(m, 1H, CH), 2.99(dd, J=17.2Hz, J=5.6Hz, 1H, cyclohexene-H), 3.56~3.65(m, 2H, cyclohexene 6-H) , 4.08(dd, J=11.6Hz, J=9.2Hz, 1H, cyclohexene-H), 4.25~4.31(m, 2H, CH2), 4.35(d, J=8.8Hz, 1H, cyclohexene- H), 4.80 (bs, HOD), 6.88 (s, 1H, cyclohexene 2-ene-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com