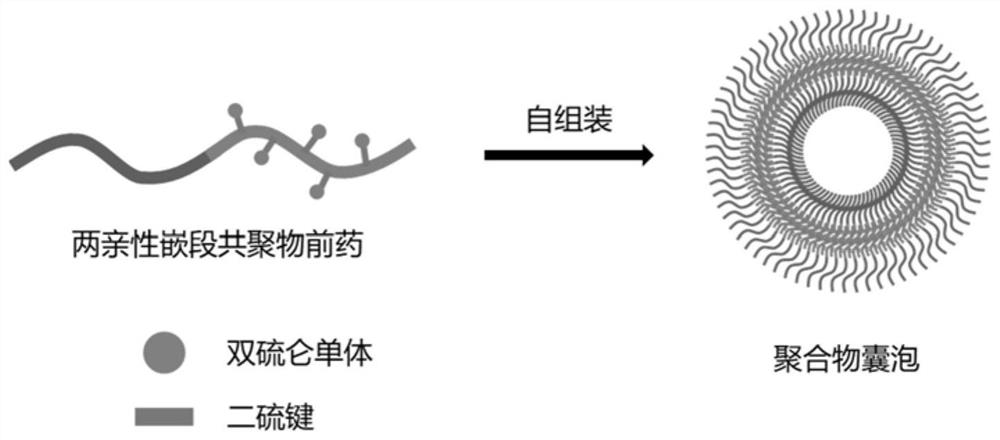
Amphiphilic block copolymer prodrug based on disulfiram as well as preparation method and application of amphiphilic block copolymer prodrug
A technology of amphiphilic block and disulfiram, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as premature release, and avoid premature release , Improve the effect of tumor therapy, improve solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
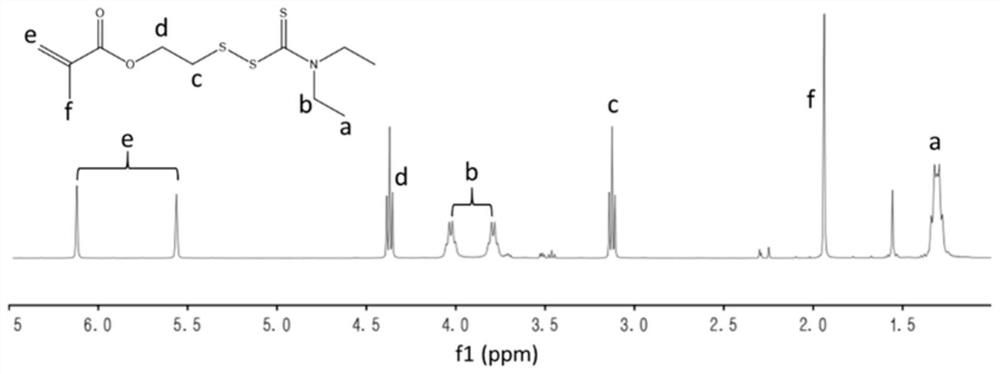
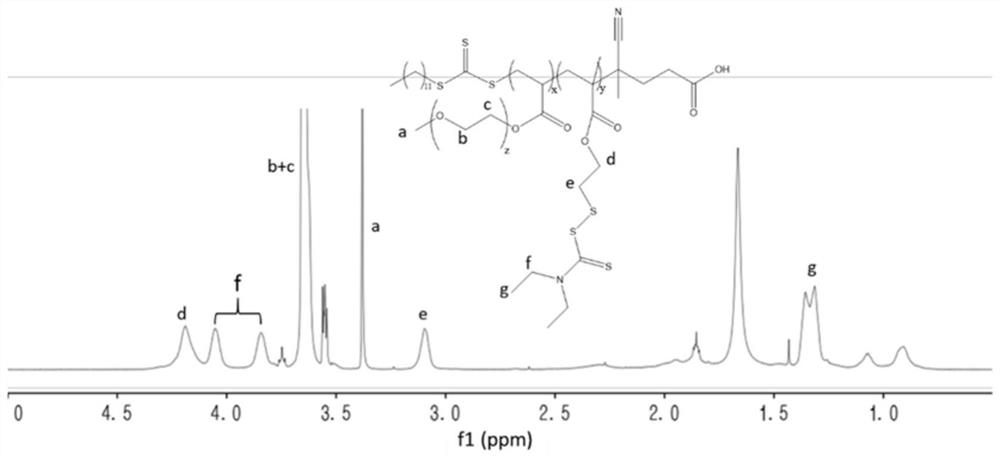
[0053] The synthesis of embodiment 1 disulfiram prodrug monomer DTCM
[0054] Weigh 2.19g of diethylamine (30.0mmol) and 2.34g of mercaptoethanol (30.0mmol) into a single-necked flask, add 30.0mL of anhydrous dichloromethane, and ice-bath. 2.28g of carbon disulfide (30.0mmol), 3.03g of triethylamine (30.0mmol) and 9.94g of carbon tetrabromide (30.0mmol) were added in turn, and the reaction was stirred at room temperature for 2h. After the reaction, it was washed with water three times and dried overnight with anhydrous sodium sulfate. Concentrate by rotary evaporation, and then purify by column chromatography using n-hexane / ethyl acetate as eluent to obtain a yellow oily product named HDTC.
[0055] Weigh 1.0g HDTC (4.4mmol) and 0.44g triethylamine (4.4mmol) into a single-necked flask, add 20.0mL of anhydrous dichloromethane, and ice-bath. Add 0.46 g of methacryloyl chloride (4.4 mmol), and stir the reaction at room temperature for 12 h. After the reaction, it was washed th...
Embodiment 2
[0056] The synthesis of embodiment 2 disulfiram prodrug monomer DTCM
[0057] Weigh 2.19g of diethylamine (30.0mmol) and 2.34g of mercaptoethanol (30.0mmol) into a single-necked flask, add 30.0mL of anhydrous dichloromethane, and ice-bath. 2.28g of carbon disulfide (30.0mmol), 3.03g of triethylamine (30.0mmol) and 14.92g of carbon tetrabromide (45.0mmol) were successively added, and the reaction was stirred at room temperature for 2h. After the reaction, it was washed with water three times and dried overnight with anhydrous sodium sulfate. Concentrate by rotary evaporation, and then purify by column chromatography using n-hexane / ethyl acetate as eluent to obtain a yellow oily product named HDTC.
[0058] Weigh 1.0g HDTC (4.4mmol) and 0.67g triethylamine (6.6mmol) into a single-necked flask, add 20.0mL of anhydrous dichloromethane, and ice-bath. Add 0.69 g of methacryloyl chloride (6.6 mmol), and stir the reaction at room temperature for 12 h. After the reaction, it was was...
Embodiment 3
[0059] The synthesis of embodiment 3 disulfiram prodrug monomer DTCM
[0060] Weigh 2.19g of diethylamine (30.0mmol) and 2.34g of mercaptoethanol (30.0mmol) into a single-necked flask, add 30.0mL of anhydrous dichloromethane, and ice-bath. 2.28g of carbon disulfide (30.0mmol), 3.03g of triethylamine (30.0mmol) and 19.89g of carbon tetrabromide (60.0mmol) were added in turn, and the reaction was stirred at room temperature for 2h. After the reaction, it was washed with water three times and dried overnight with anhydrous sodium sulfate. Concentrate by rotary evaporation, and then purify by column chromatography using n-hexane / ethyl acetate as eluent to obtain a yellow oily product named HDTC.
[0061] Weigh 1.0g HDTC (4.4mmol) and 0.89g triethylamine (8.8mmol) into a single-necked flask, add 20.0mL of anhydrous dichloromethane, and ice-bath. Add 0.92 g of methacryloyl chloride (8.8 mmol), and stir the reaction at room temperature for 12 h. After the reaction, it was washed t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com