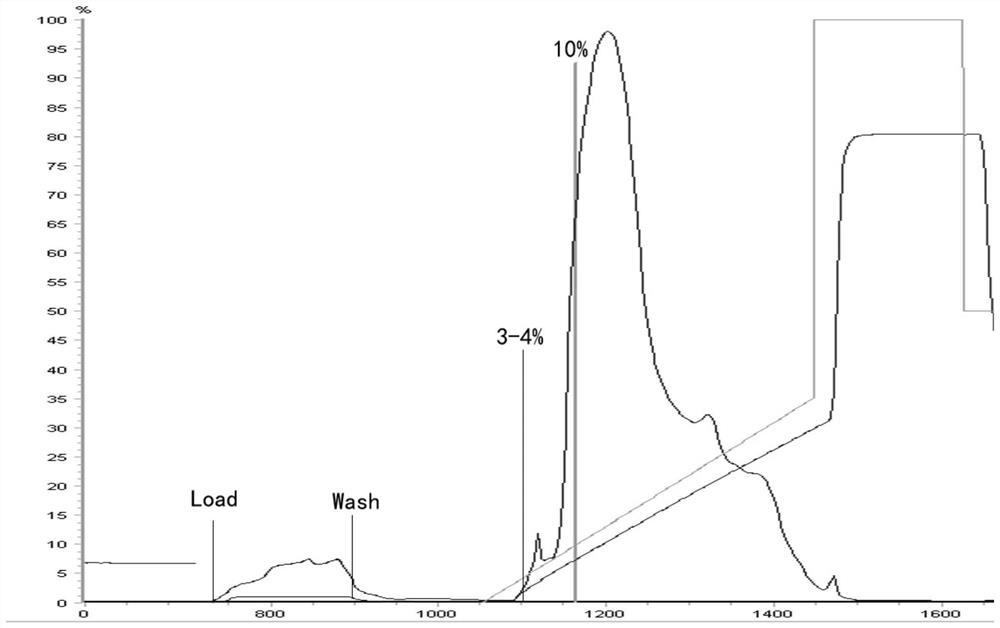
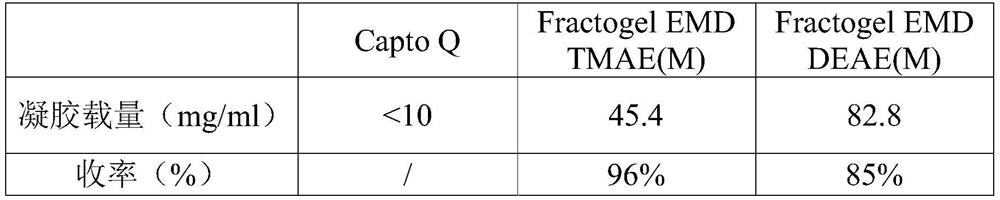
Purification method of human fibrinogen
A fibrinogen and buffer technology, applied in the field of purification of human fibrinogen, can solve the problems of complicated purification steps and residual virus inactivator tributyl phosphate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1. Purification method of human fibrinogen
[0039] Step 1: Preliminary Purification
[0040] 1. Take the cryoprecipitate prepared from fresh frozen plasma, and dissolve the cryoprecipitate in 20mM Tris buffer solution with a pH of 6.8 at a weight ratio of 1:3 at room temperature to obtain a cryoprecipitate solution;
[0041] 2. Add aluminum hydroxide (the amount of aluminum hydroxide is 2wt.% of the cryoprecipitate) to the cryoprecipitate solution, and stir at room temperature for 15 minutes to carry out adsorption treatment;
[0042] 3. Centrifuge the system obtained after adsorption at 4000g for 20 minutes, take the supernatant and pass it through a 0.45μm filter membrane, and keep the filtrate;
[0043] Step 2: Virus inactivation
[0044] Carry out virus inactivation to gained filtrate by S / D method: get the first step gained filtrate, add polysorbate 80 (polysorbate 80 consumption is the 1wt.% of filtrate) and tributyl phosphate (tributyl phosphate consu...
experiment example 1
[0050] Experimental example 1. Adsorption effect evaluation
[0051] 1. Experimental method
[0052] The cryoprecipitate in the first step of Example 1 and the filtrate after the adsorption treatment were respectively used as test samples to test the purity of human fibrinogen and the adsorption effects of coagulation factors II, VII, IX, and X in the samples before and after preliminary purification.
[0053] 2. Experimental results
[0054] Table 1 Human fibrinogen and adsorbed coagulation factors II, VII, IX, X (n=3) in samples before and after preliminary purification
[0055] Test indicators Before initial purification After initial purification Protein content mg / ml (n=8) 34.65 33.01 Coagulable protein content mg / ml (n=8) 21.88 21.49 Purity % (n=8) 63.15 65.10 FII IU / ml (n=2) 0.32 0.11 FVII IU / ml (n=2) 0.54 0.08 FIX IU / ml (n=2) 0.85 0.23 FX IU / ml (n=2) 0.13 0.04
[0056] In Table 1, the protein content ...
experiment example 2
[0058] Experimental example 2. Evaluation of virus inactivation effect
[0059] 1. Experimental method
[0060] The sample to be loaded on the column obtained after the virus inactivation in the second step of Example 1 was used as the test sample to evaluate the virus inactivation effect.
[0061] 2. Experimental results
[0062] Table 2. SD virus inactivation effect (n=3)
[0063] indicator virus PRV virus Sindbis virus HIV virus Inactivation effect ≥4.75logTCID 50 / ml
[0064] As can be seen from the results in Table 2, carrying out SD virus inactivation with the method of the present invention can effectively inactivate lipid-enveloped viruses and reach the national standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com