Patents
Literature
42 results about "Plasma products" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasma and Recombinant Products. A range of valuable products are manufactured from blood plasma through a process called fractionation, in which different types of proteins found in blood plasma are separated, purified and concentrated into therapeutic doses.
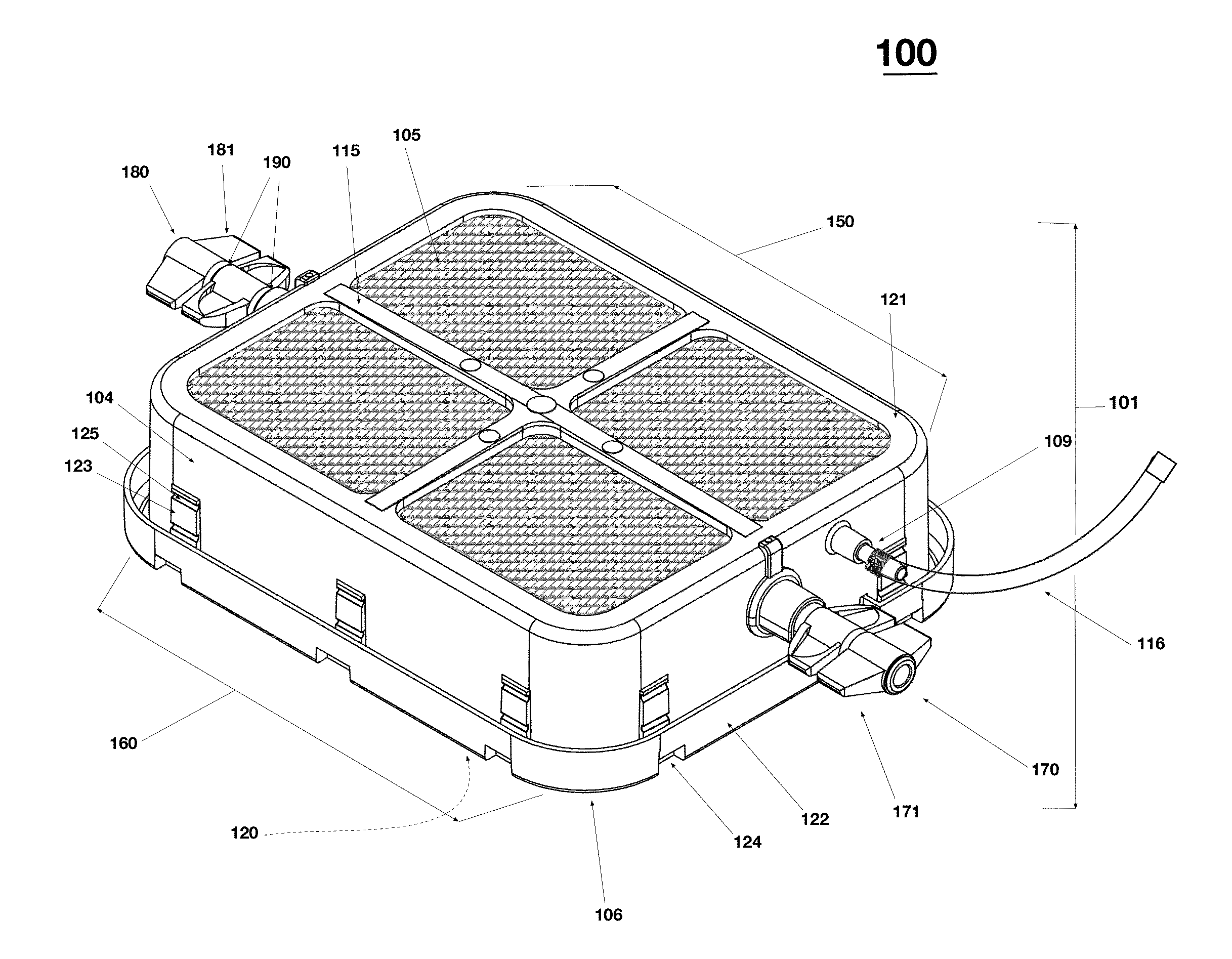
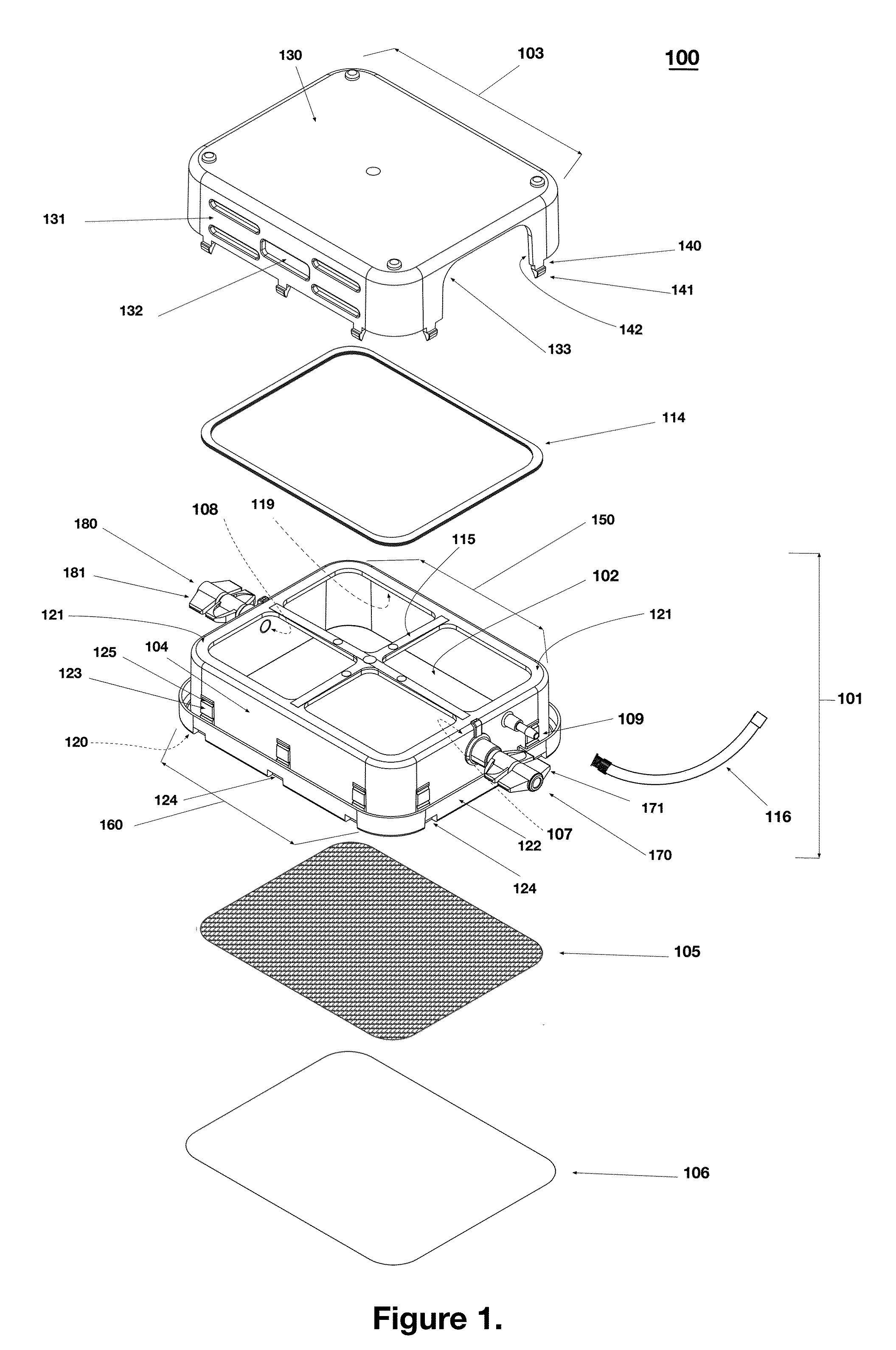
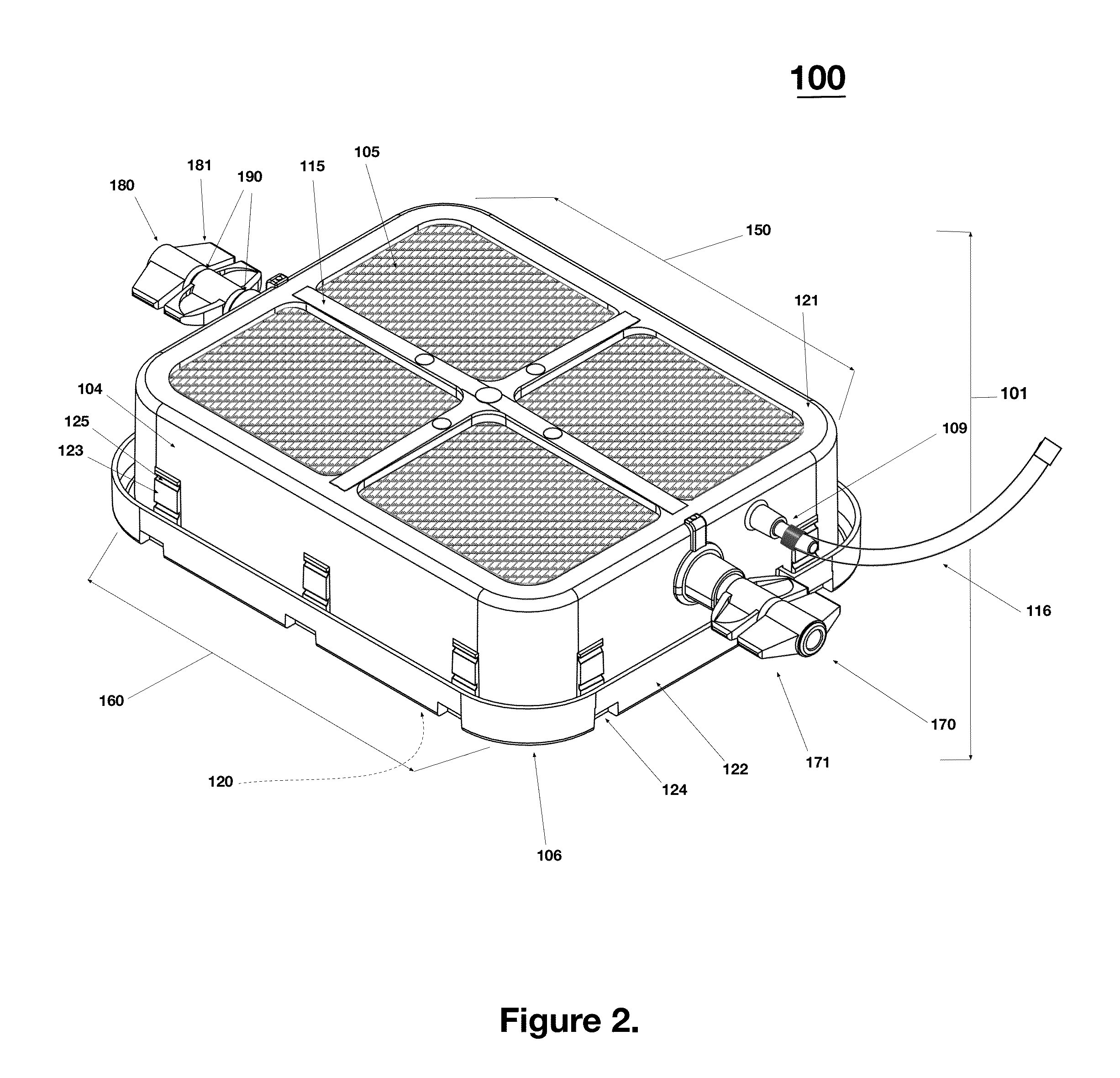
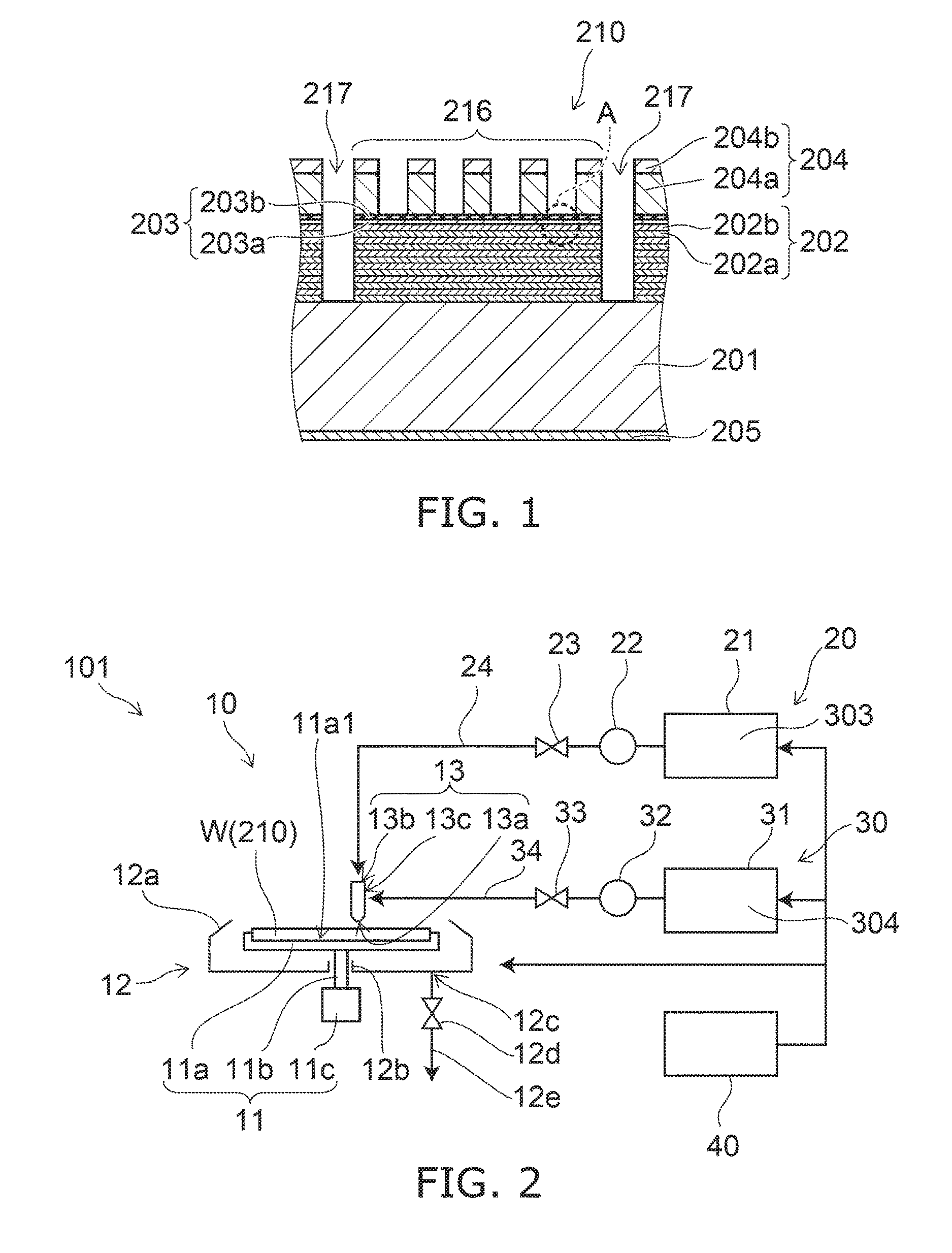
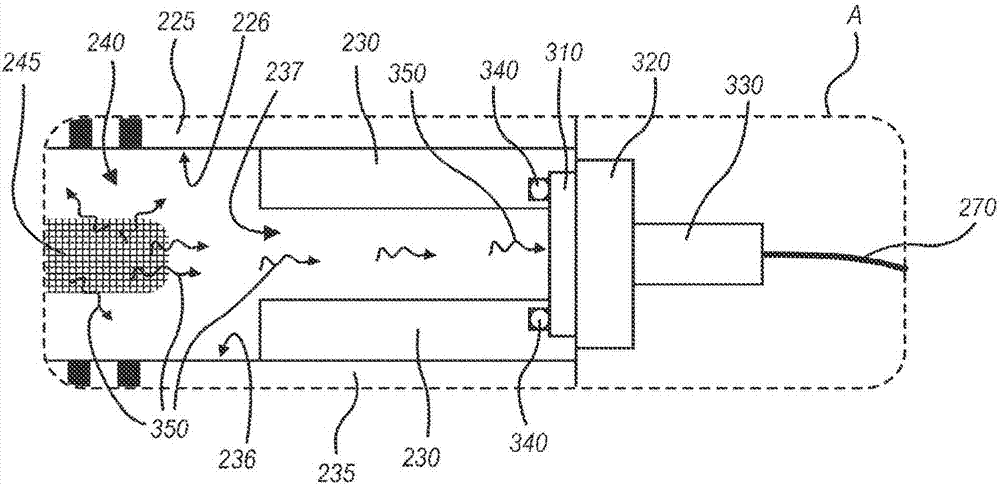
Low Aspect Ratio Staged Closure Devices, Systems, and Methods for Freeze-Drying, Storing, Reconstituting, and Administering Lyophilized Plasma
InactiveUS20140259724A1Easy to keepAvoid cross contaminationDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingEngineering
The inventive device and methods described herein address the introduction of a safe and effective freeze-dried biological product, and particularly a plasma product, to a subject in need thereof. The present invention relates to a multifunctional, staged closure device, which also is described as a lyophilization container for plasma (LCP). The device and methods described herein address how to reproducibly achieve a low moisture and substantially oxygen-free atmosphere within a finally hermetically sealed biocompatible low aspect plastic vessel within a standard shelf-stoppering freeze dryer. The present inventive device and methods provide a freeze-dried plasma product that is fully traceable, preserves the constituent plasma activity, is readily prepared in a sterile fashion, is stable, ensures ease of storage and permits rapid reconstitution and delivery to a patient.
Owner:HEMCON MEDICAL TECH
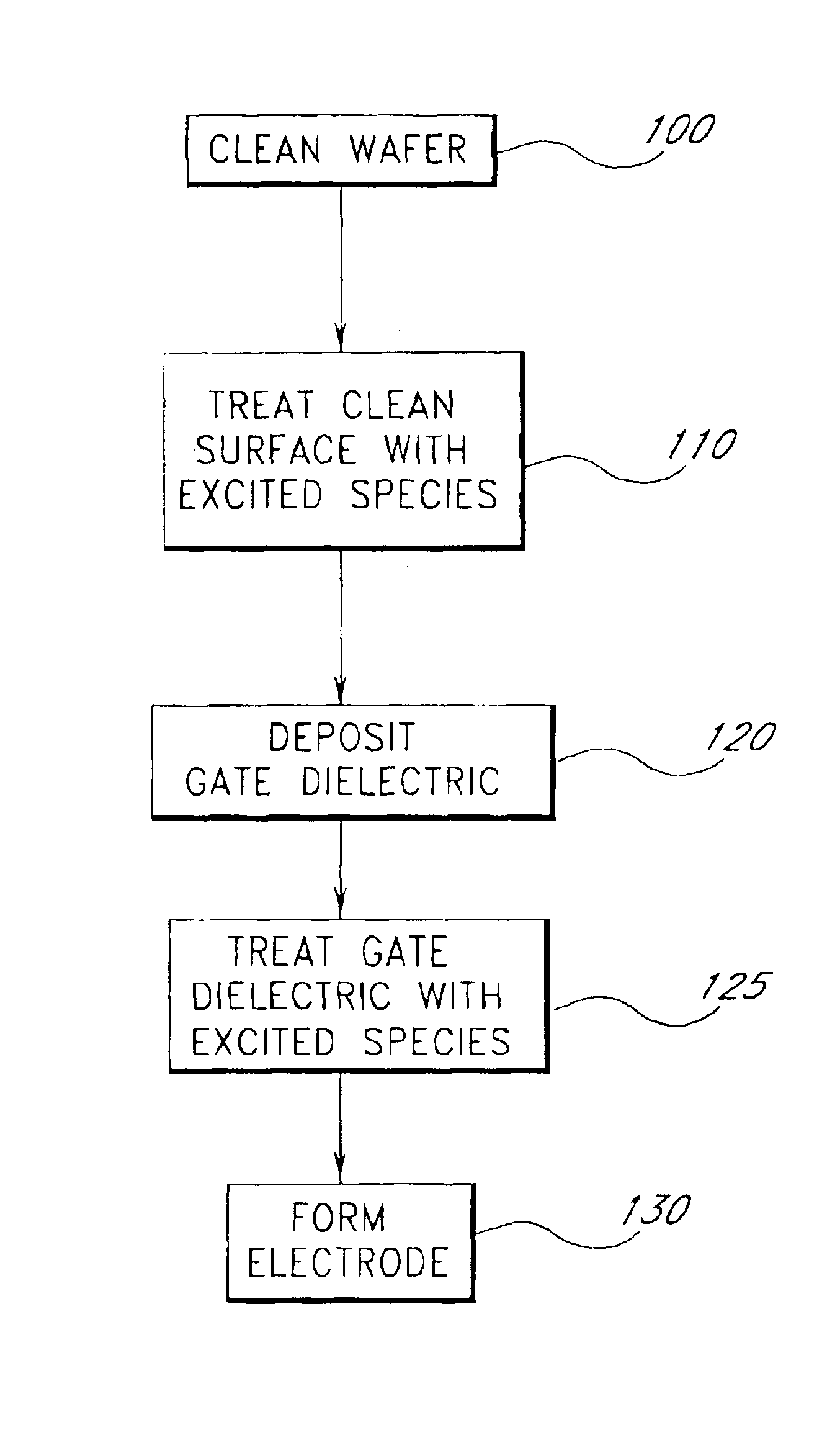
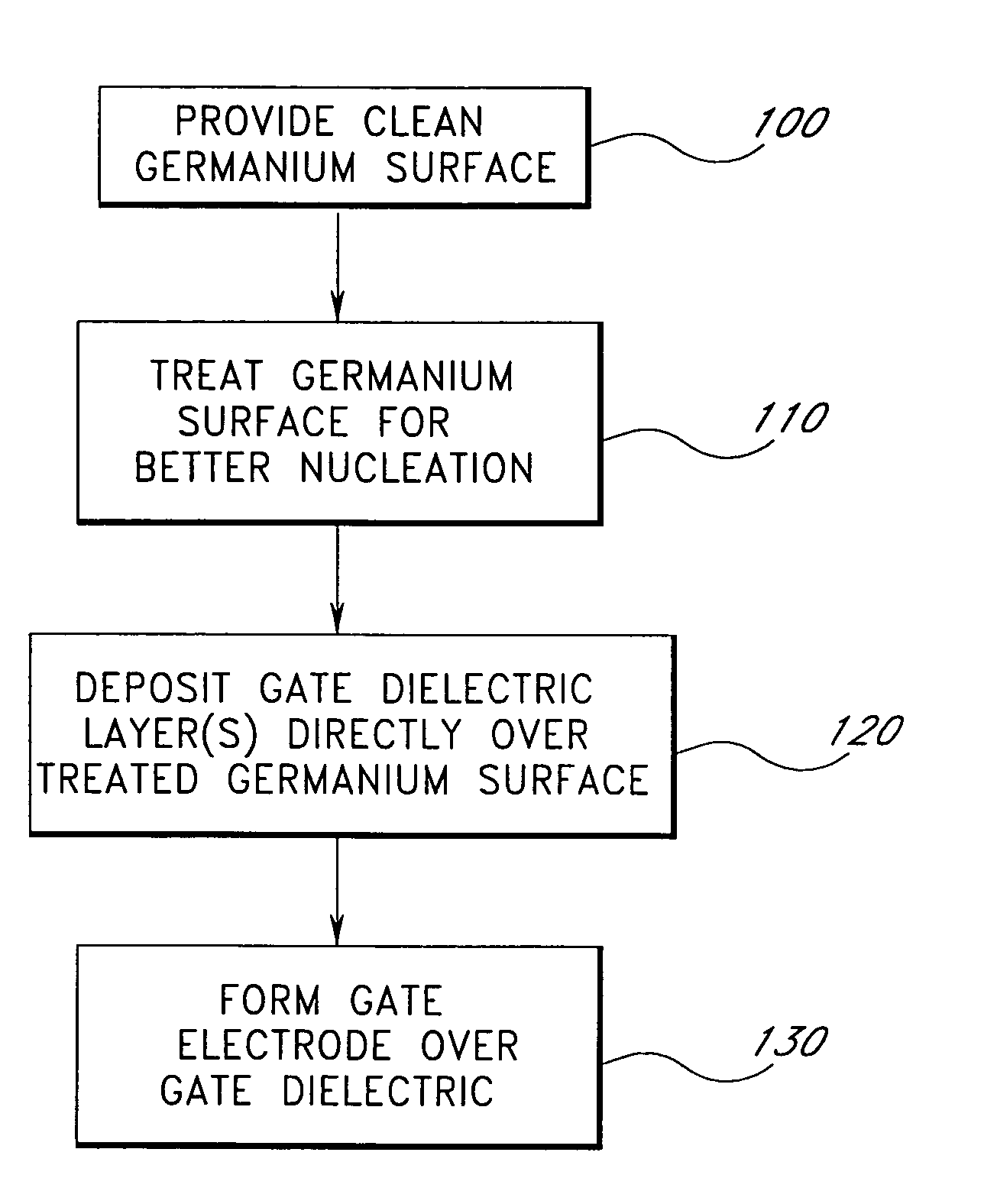
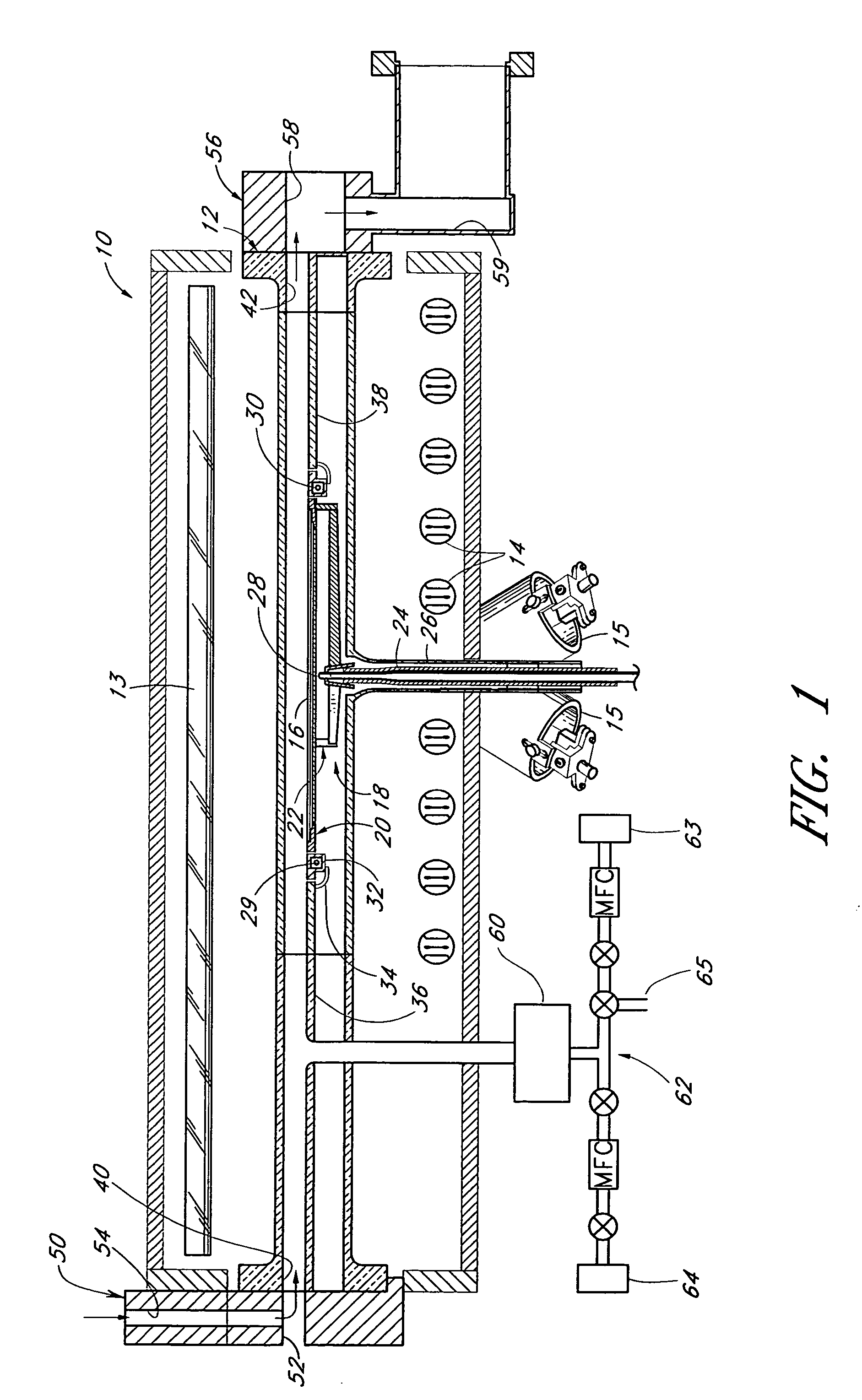
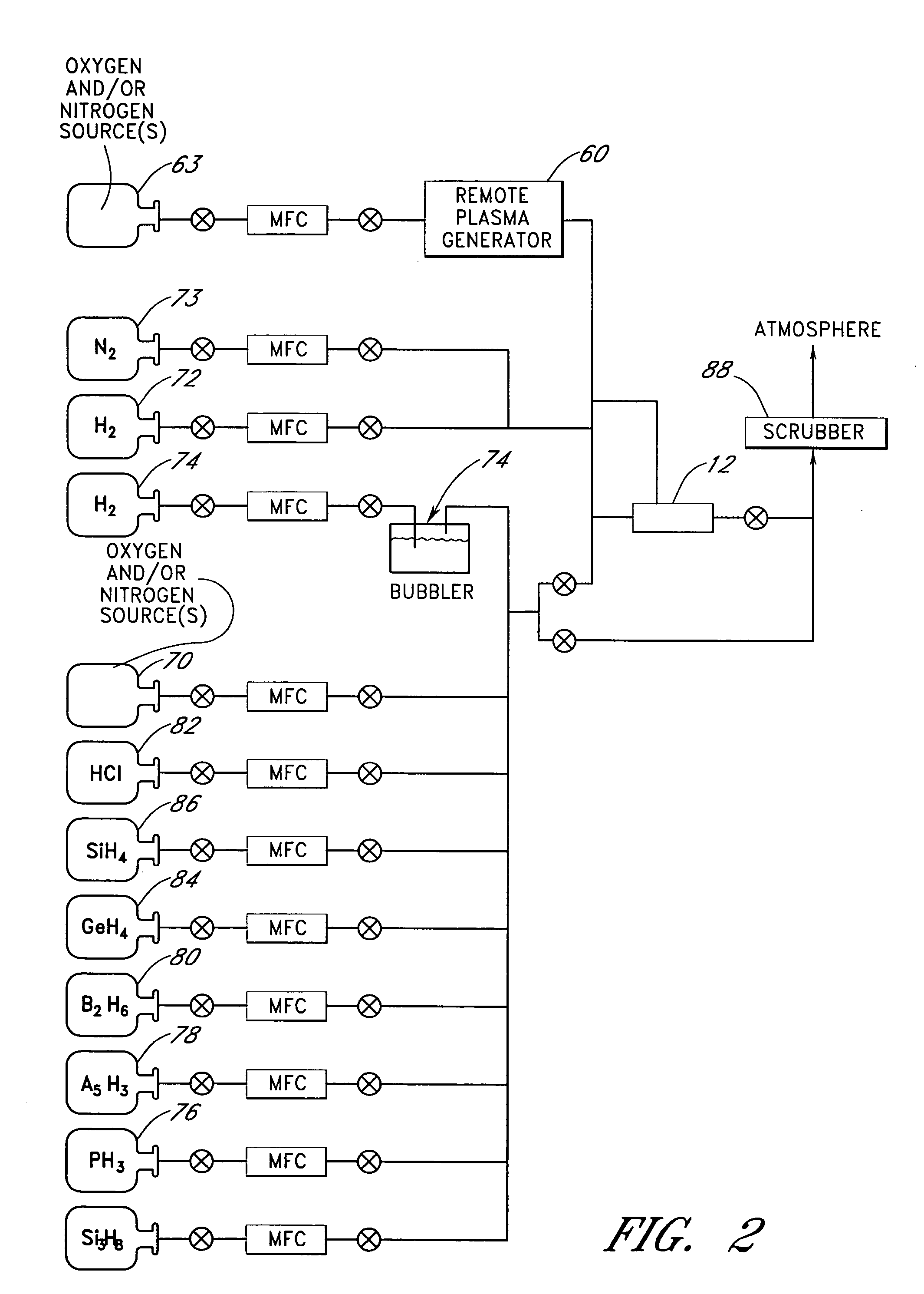
Surface preparation prior to deposition
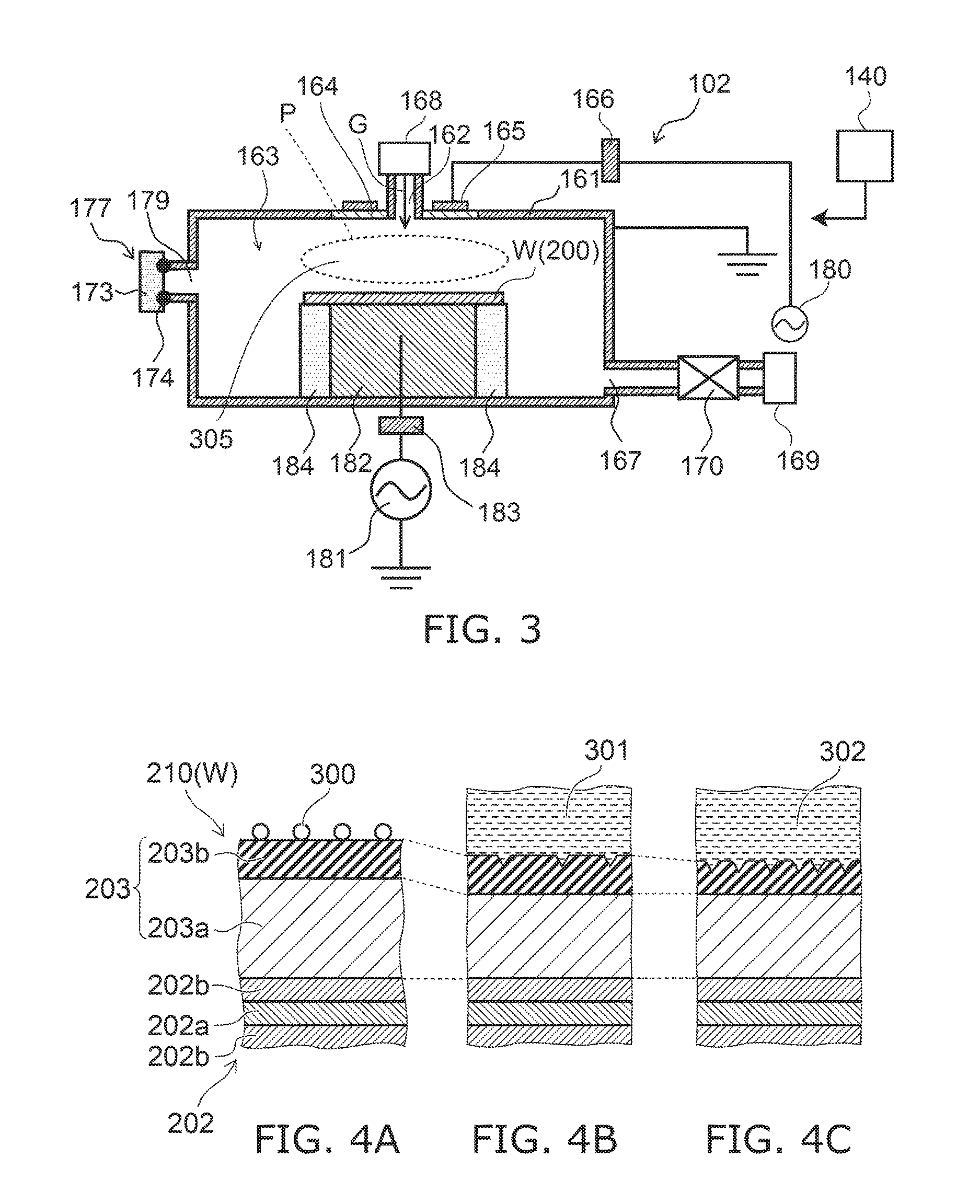
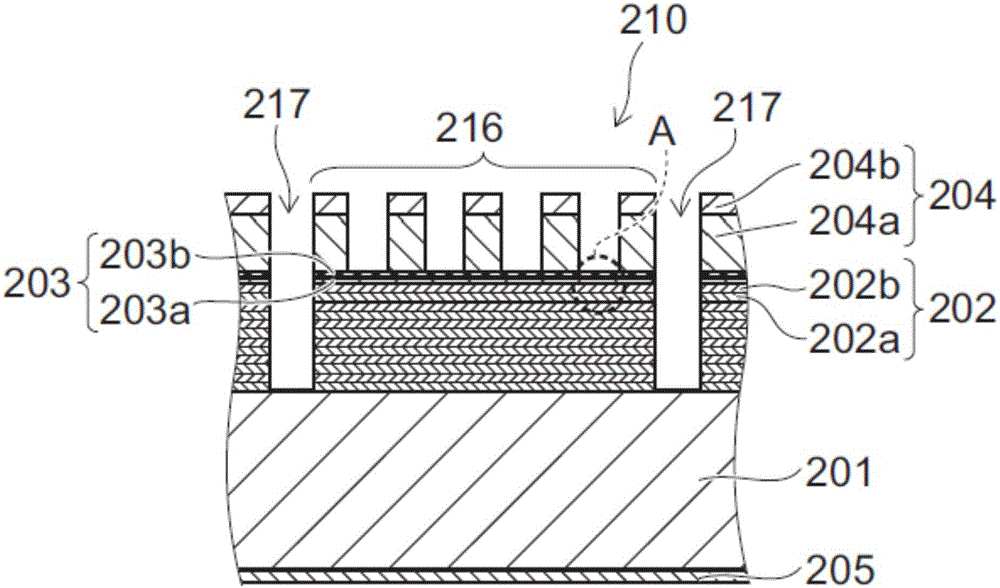
InactiveUS6958277B2Nucleation is easyWithout significantly affectingSemiconductor/solid-state device manufacturingChemical vapor deposition coatingGate dielectricAtomic layer deposition
Methods are provided herein for treating substrate surfaces in preparation for subsequent nucleation-sensitive depositions (e.g., polysilicon or poly-SiGe) and adsorption-driven deposition (e.g. atomic layer deposition or ALD). Prior to depositing, the surface is treated with non-depositing plasma products. The treated surface more readily nucleates polysilicon and poly-SiGe (such as for a gate electrode), or more readily adsorbs ALD reactants (such as for a gate dielectric). The surface treatment provides surface moieties more readily susceptible to a subsequent deposition reaction, or more readily susceptible to further surface treatment prior to deposition. By changing the surface termination of the substrate with a low temperature radical treatment, subsequent deposition is advantageously facilitated without depositing a layer of any appreciable thickness and without significantly affecting the bulk properties of the underlying material. Preferably less than 10 Å of the bulk material incorporates the excited species, which can include fluorine, chlorine and particularly nitrogen excited species.
Owner:ASM IP HLDG BV
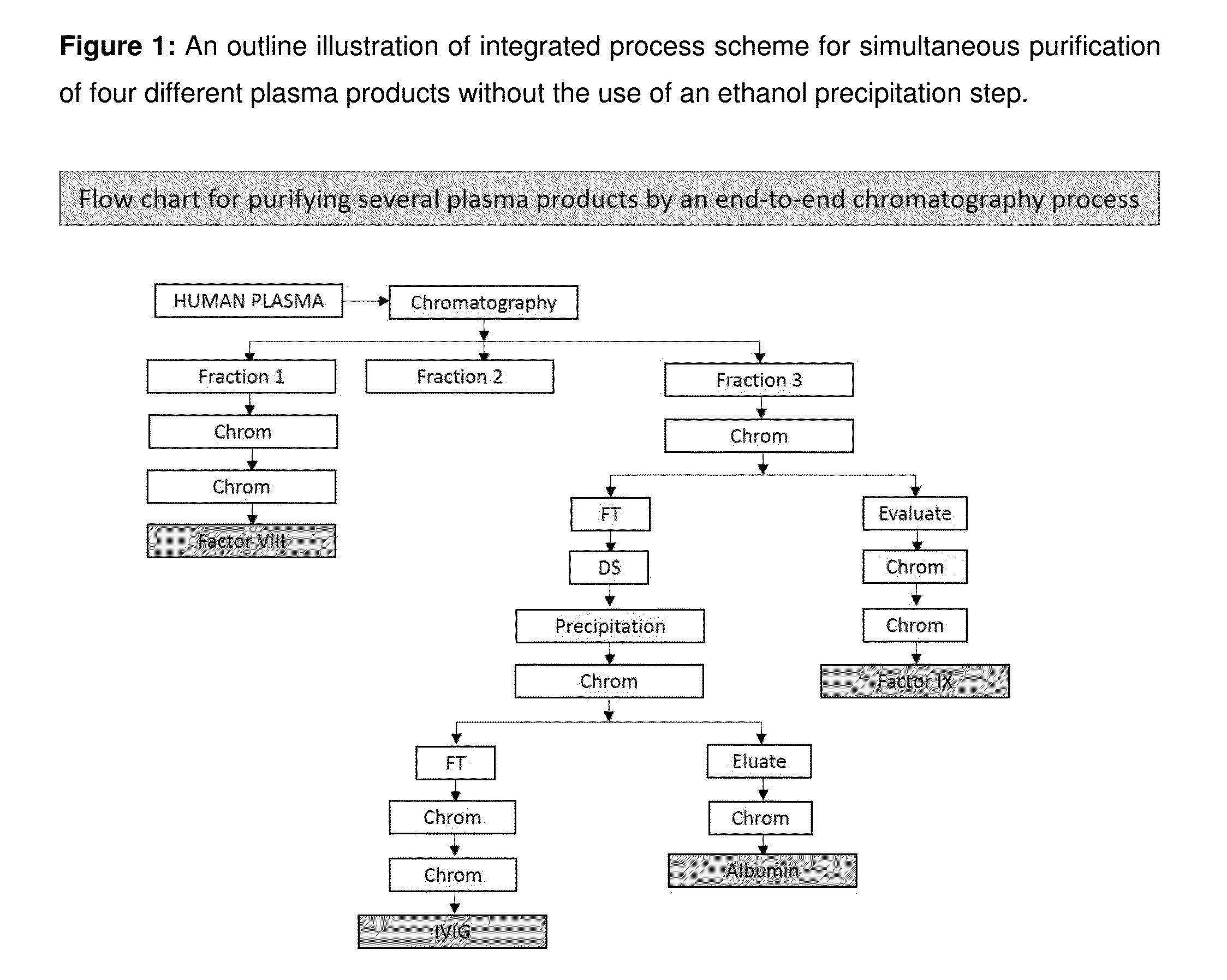
Reactor for wafer backside polymer removal using plasma products in a lower process zone and purge gases in an upper process zone
InactiveUS20080179007A1Electric discharge tubesSemiconductor/solid-state device manufacturingProcess engineeringProduct gas
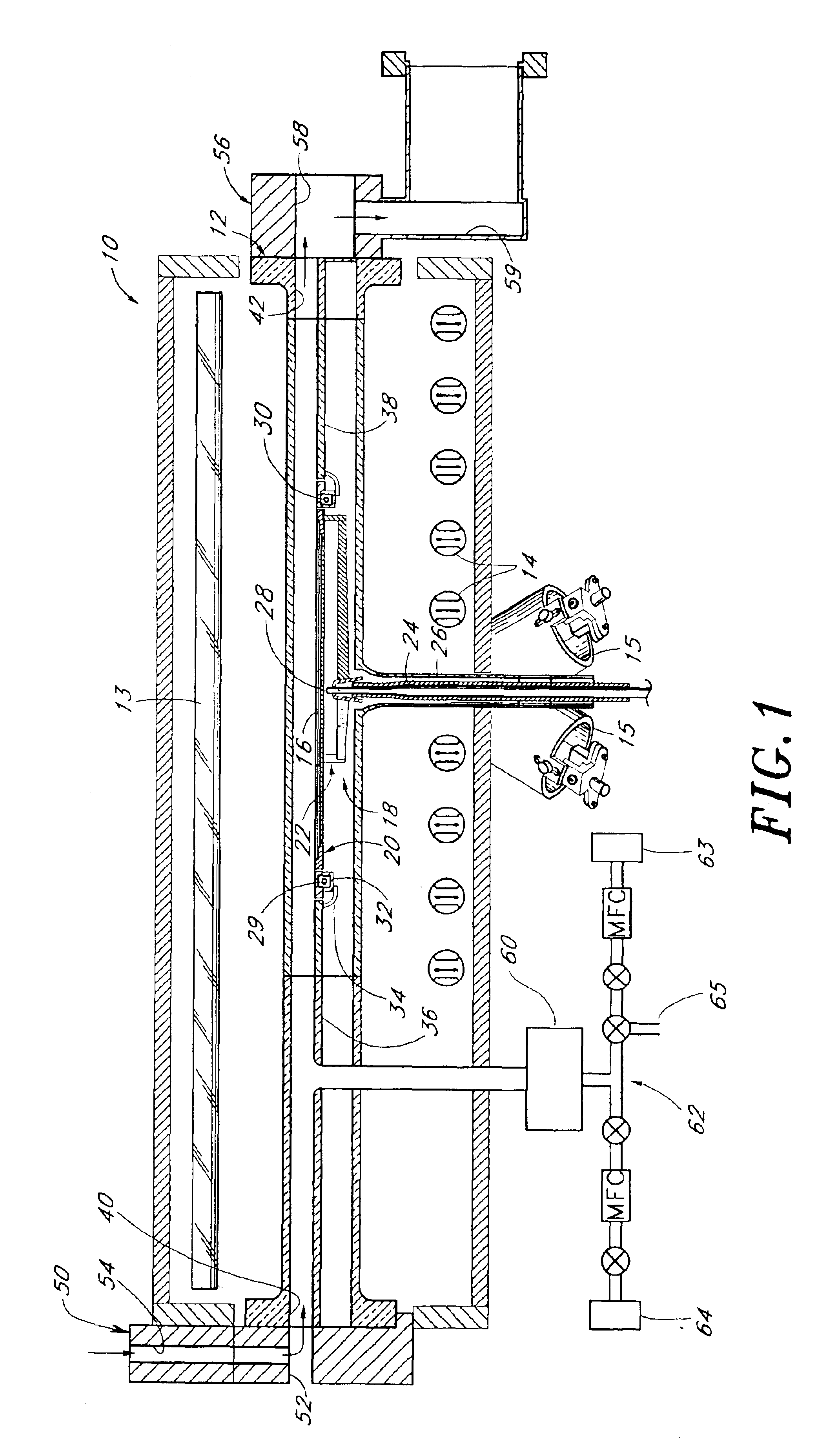
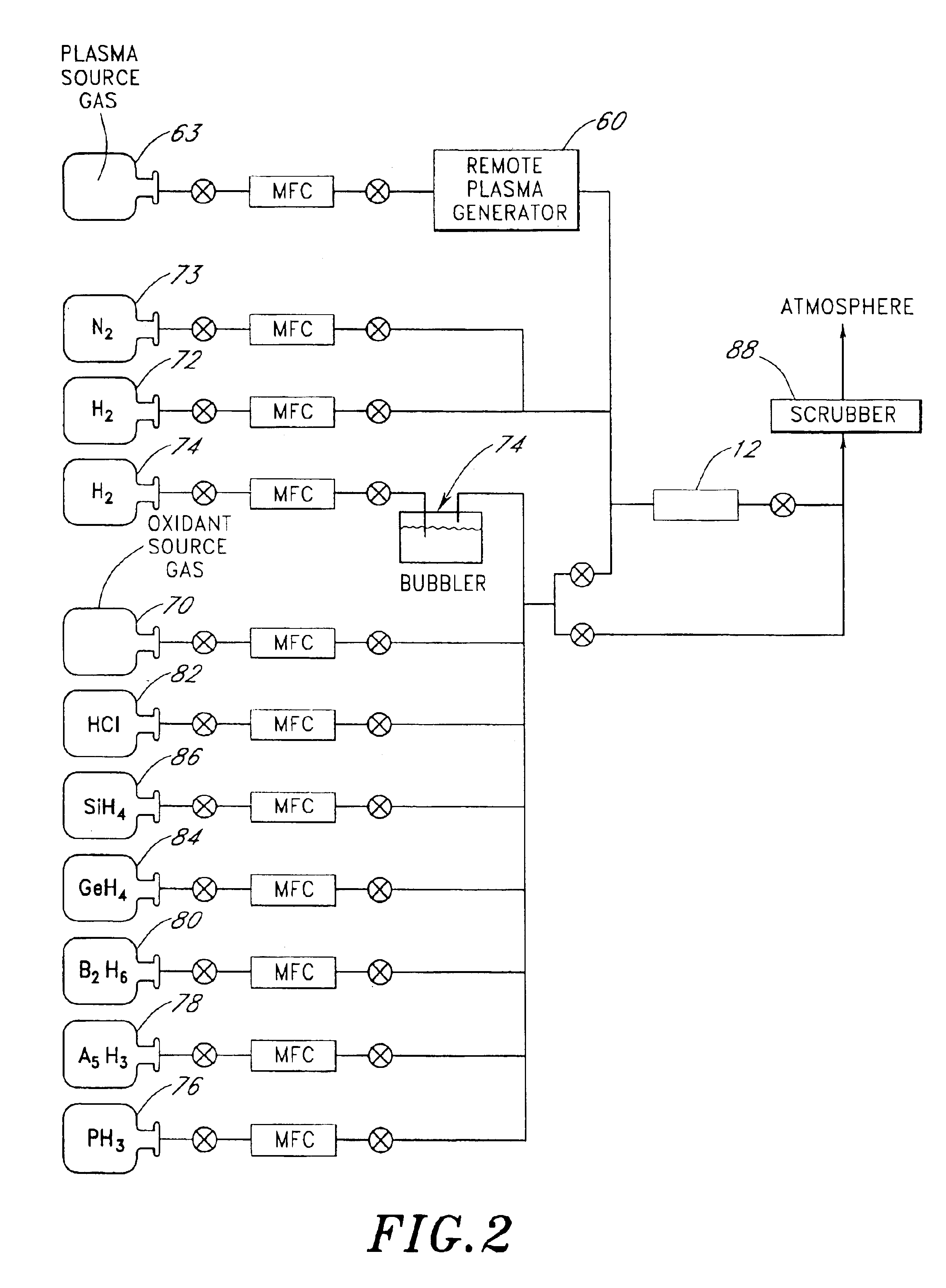
A reactor is provided for removing polymer from a backside of a workpiece. The reactor includes a vacuum chamber having a ceiling, a floor and a cylindrical side wall. The reactor further includes workpiece support apparatus within the chamber configured for a workpiece to be placed thereon with its front side facing the ceiling. The support apparatus is configured to leave at least an annular periphery of the backside of the workpiece exposed. A confinement member defines a narrow gap with an outer edge of the workpiece, the narrow gap being on the order of about 1% of the workpiece diameter, the narrow gap corresponding to a boundary dividing the chamber between an upper process zone and a lower process zone, the reactor further comprising a vacuum pump coupled to the lower process zone. An external plasma-generating chamber is coupled to the chamber, the external plasma-generating chamber configured to introduce a plasma by-product into the lower process zone, and a supply of a polymer etch precursor gas to the external plasma-generating chamber. The ceiling includes a gas distribution plate facing the upper process zone, the reactor further comprising a purge gas supply coupled to the gas distribution plate.
Owner:APPLIED MATERIALS INC
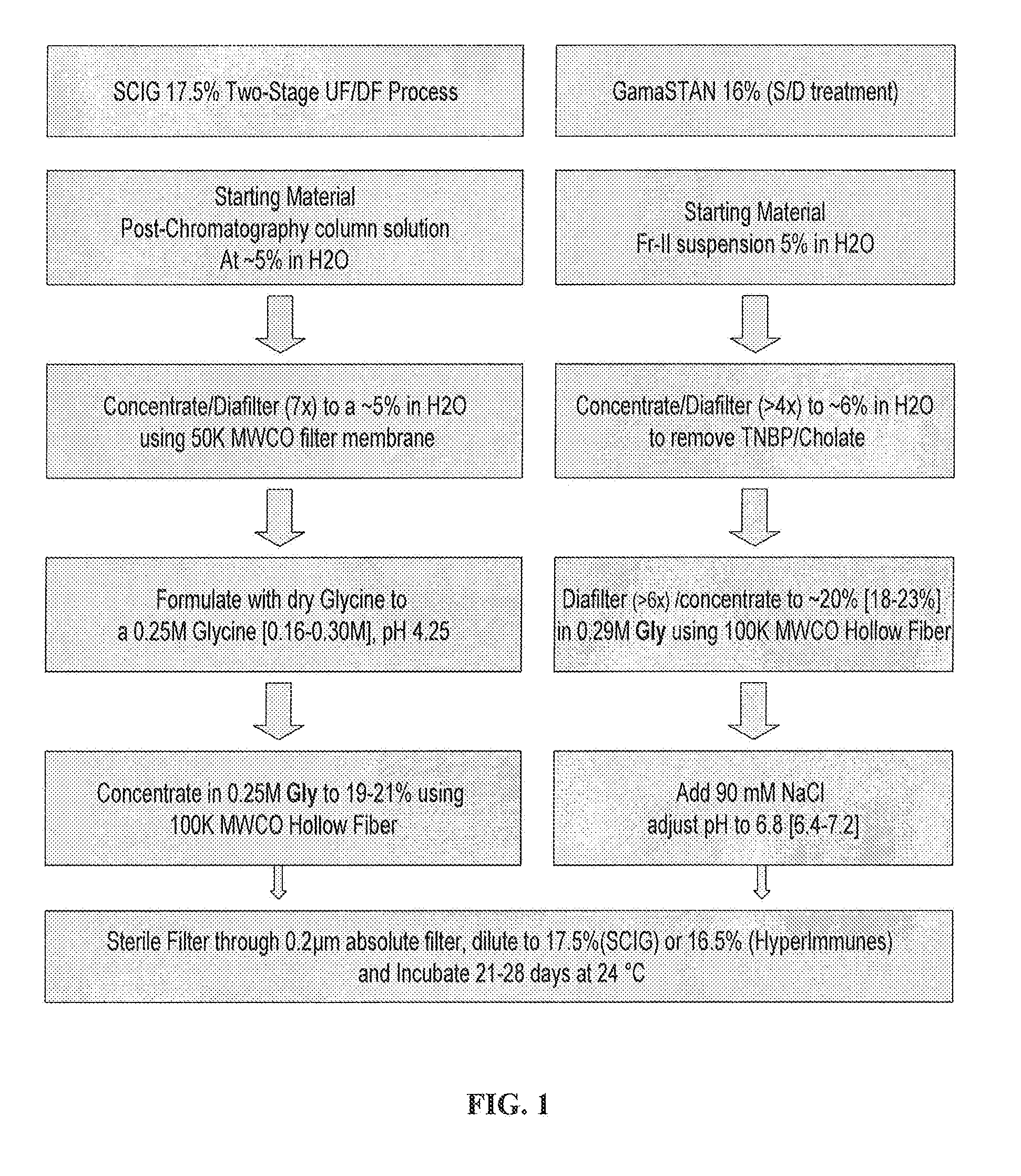
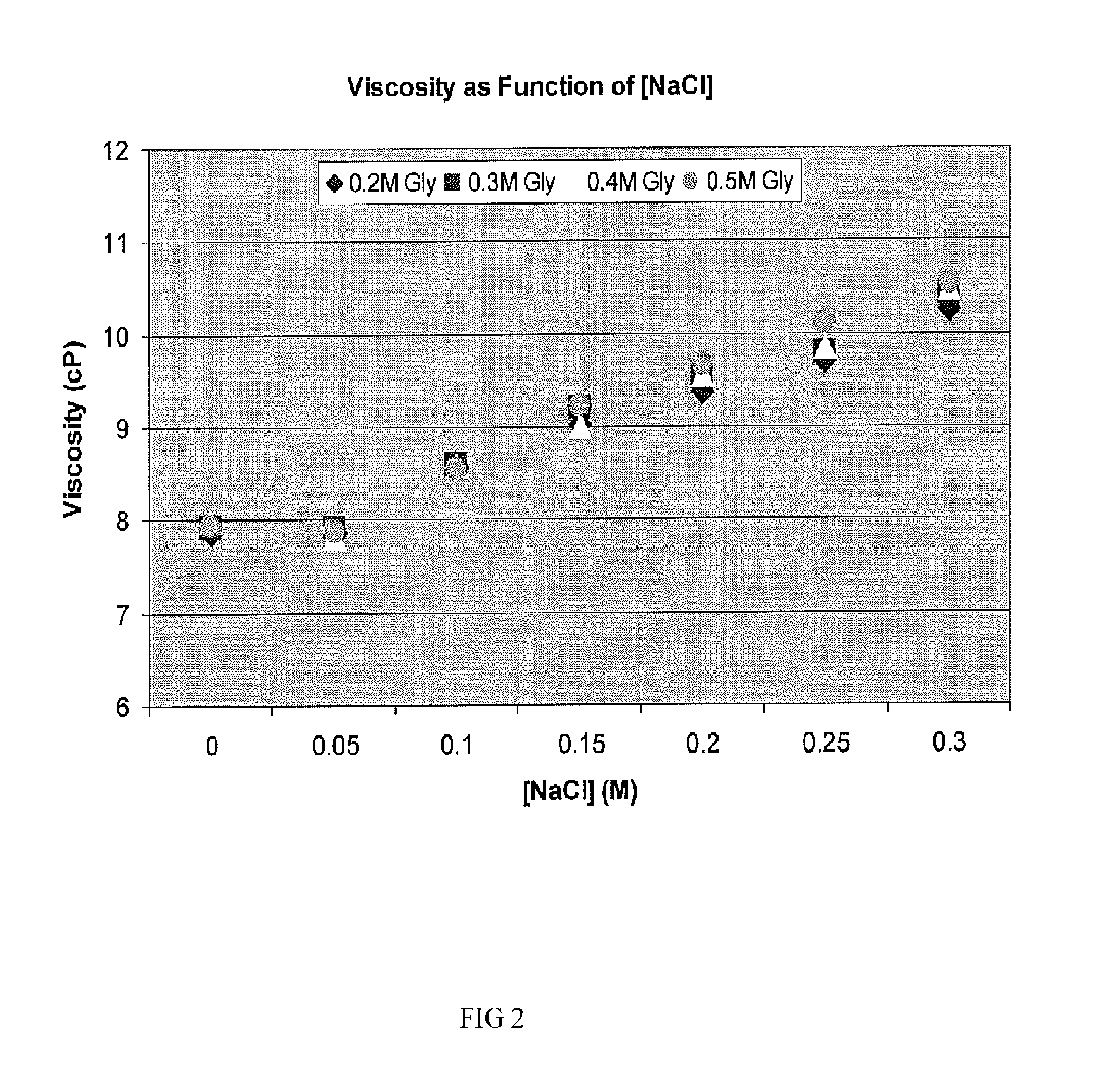
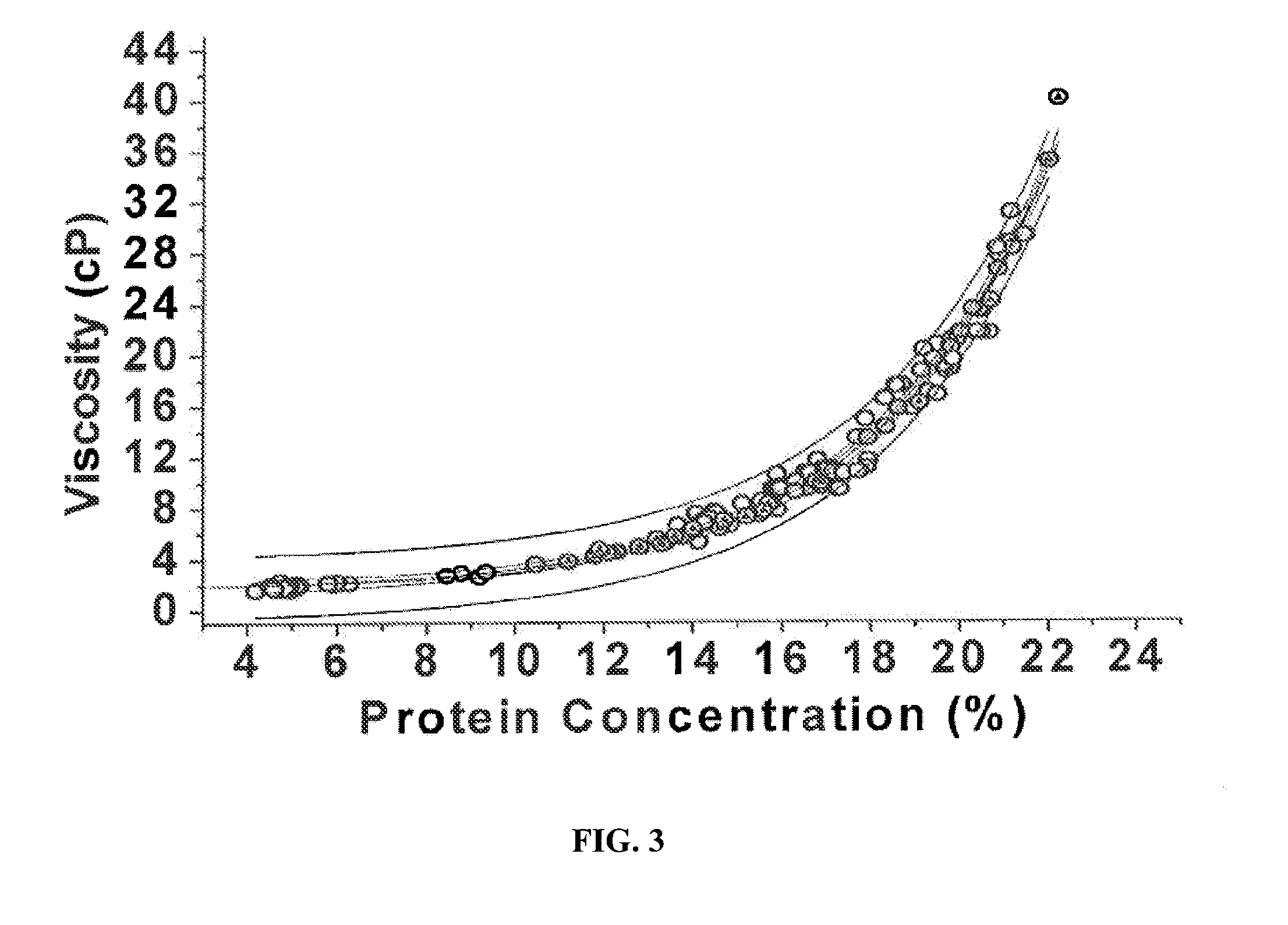
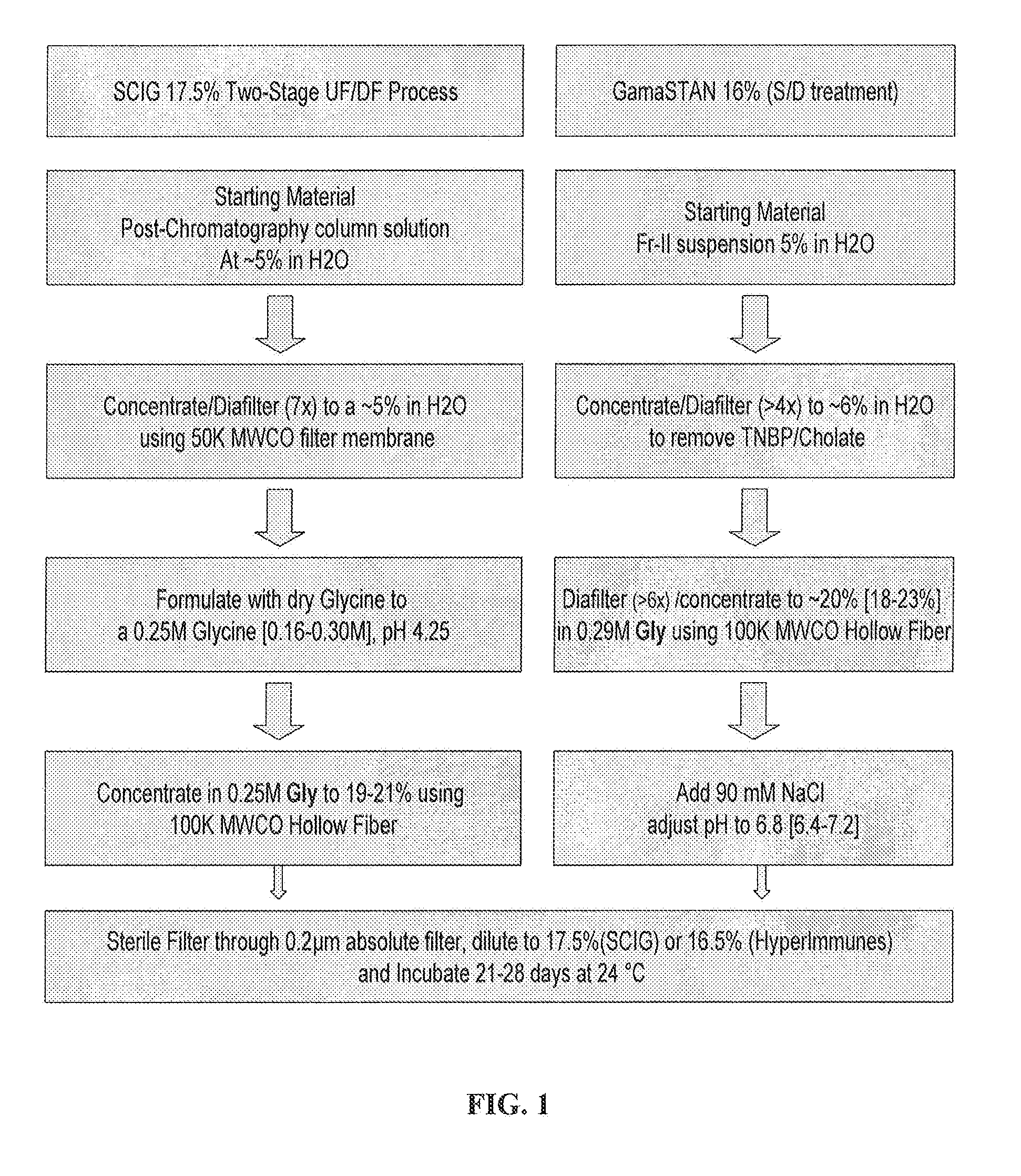
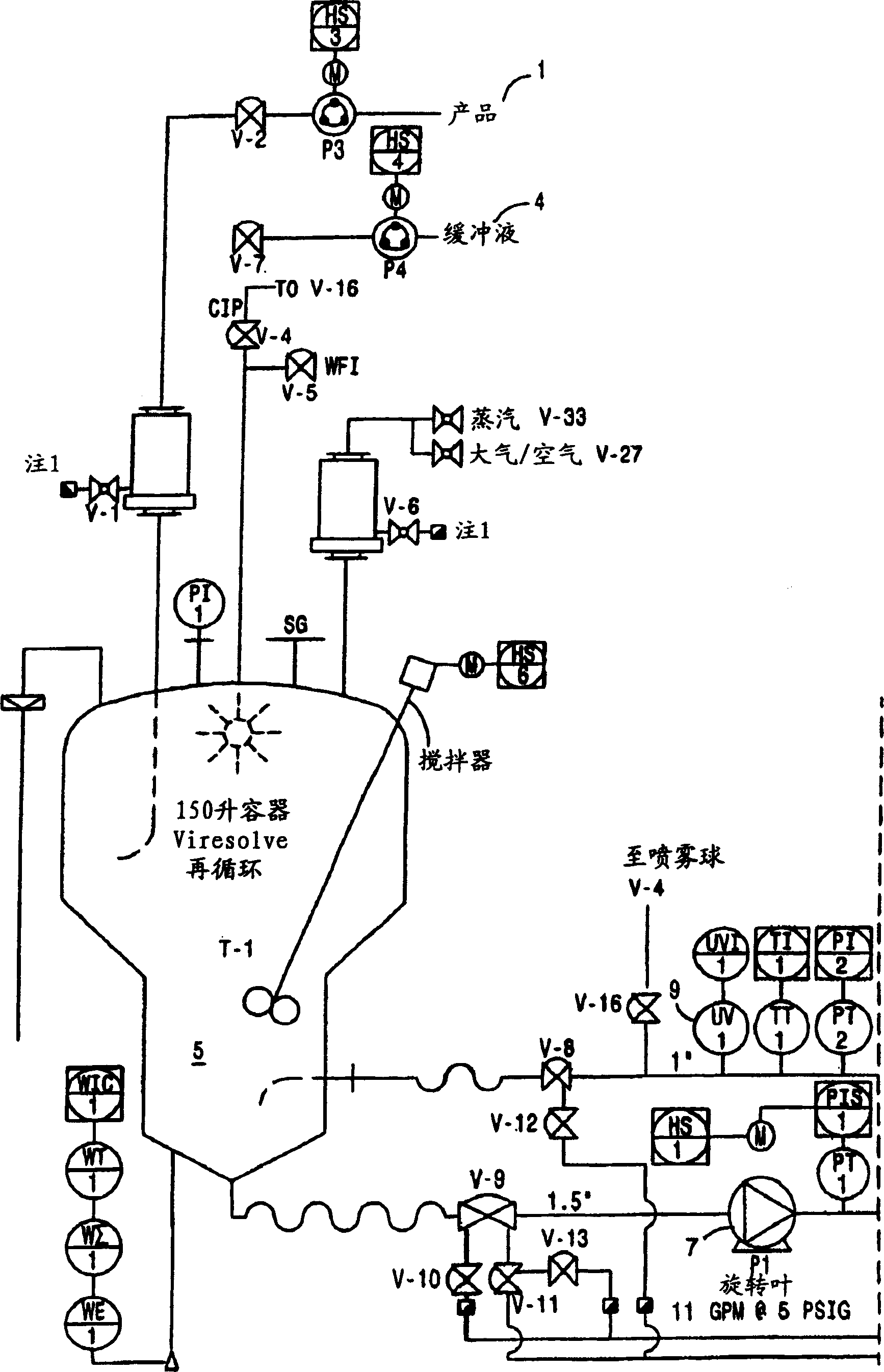
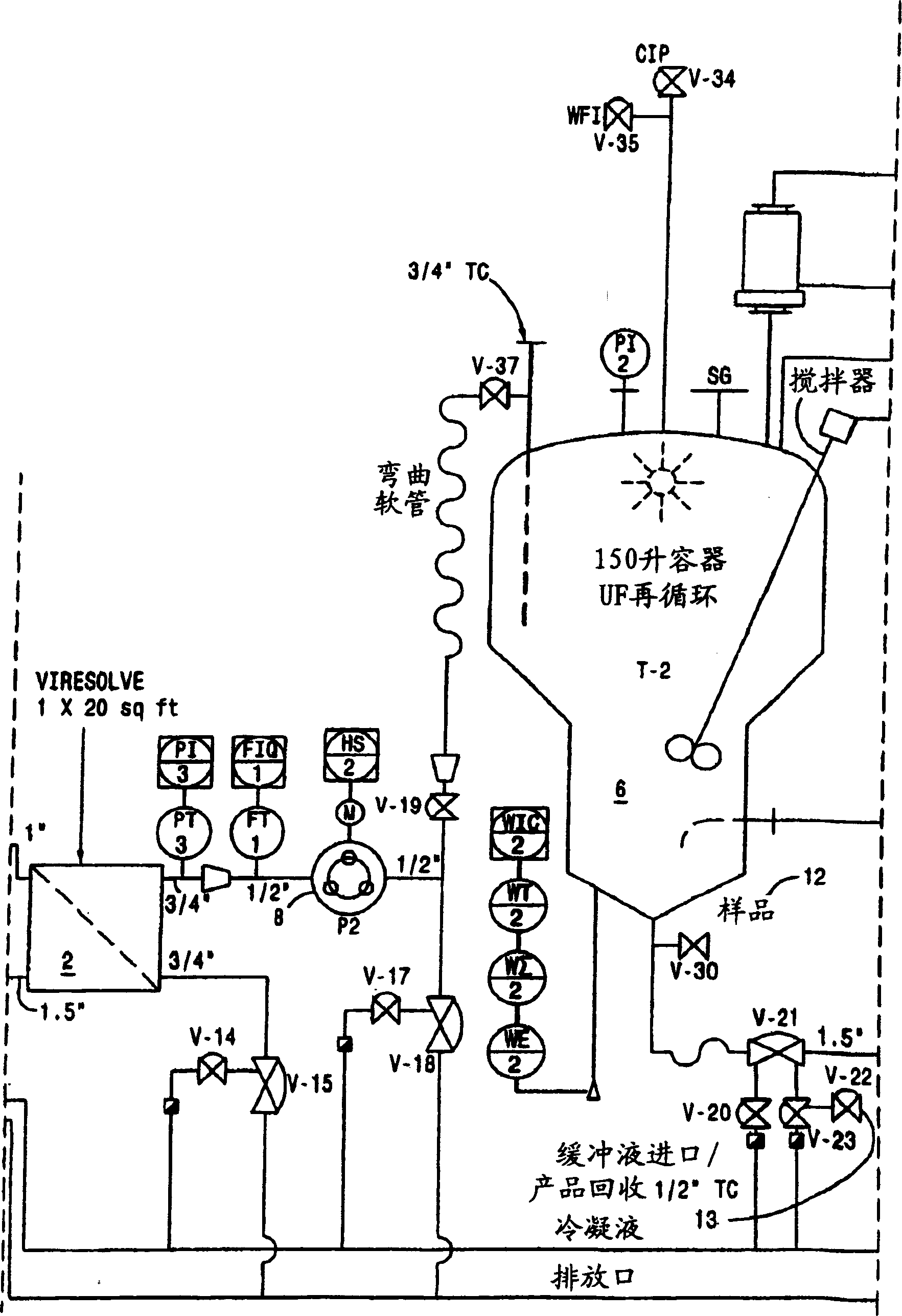
Two-stage ultrafiltration/diafiltration
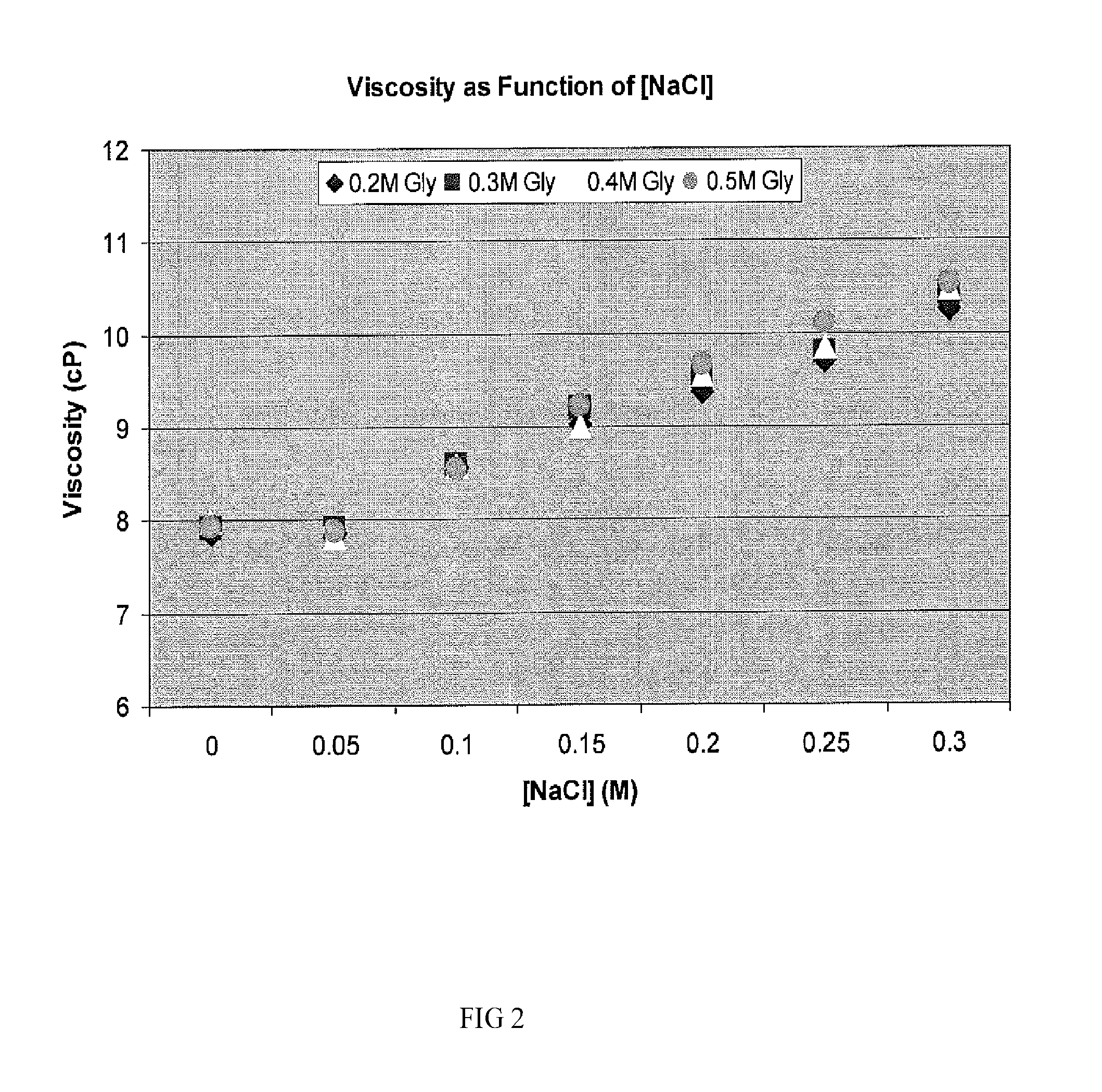
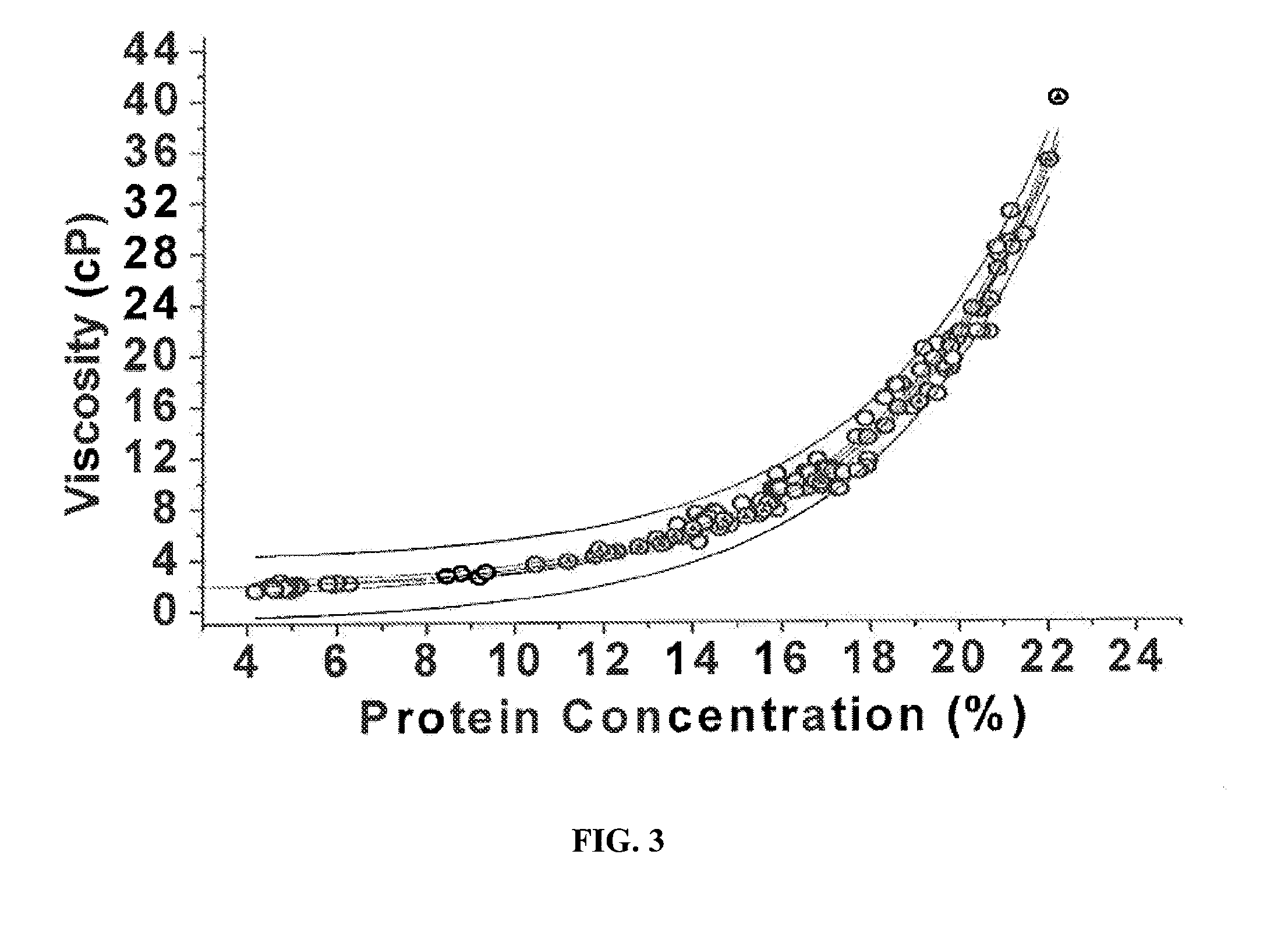
The present invention provides a method for concentrating a protein, in particular a method for concentrating a plasma product, in particular IgG, using glycine in a (two-stage ultrafiltration / diafiltration approach.
Owner:GRIFOLS THERAPEUTICS LLC
Surface preparation prior to deposition on germanium
ActiveUS7202166B2Increase speedImprove efficiencyVacuum evaporation coatingSputtering coatingGate dielectricNitrogen
Owner:ASM IP HLDG BV
Two-stage ultrafiltration/diafiltration
The present invention provides a method for concentrating a protein, in particular a method for concentrating a plasma product, in particular IgG, using glycine in a (two-stage ultrafiltration / diafiltration approach.
Owner:GRIFOLS THERAPEUTICS LLC
Plasma treatment apparatus and method
InactiveUS20070163499A1Increase temperatureLess-effective surface treatmentUltrasonic/sonic fibre treatmentEnergy based chemical/physical/physico-chemical processesInstabilityEngineering
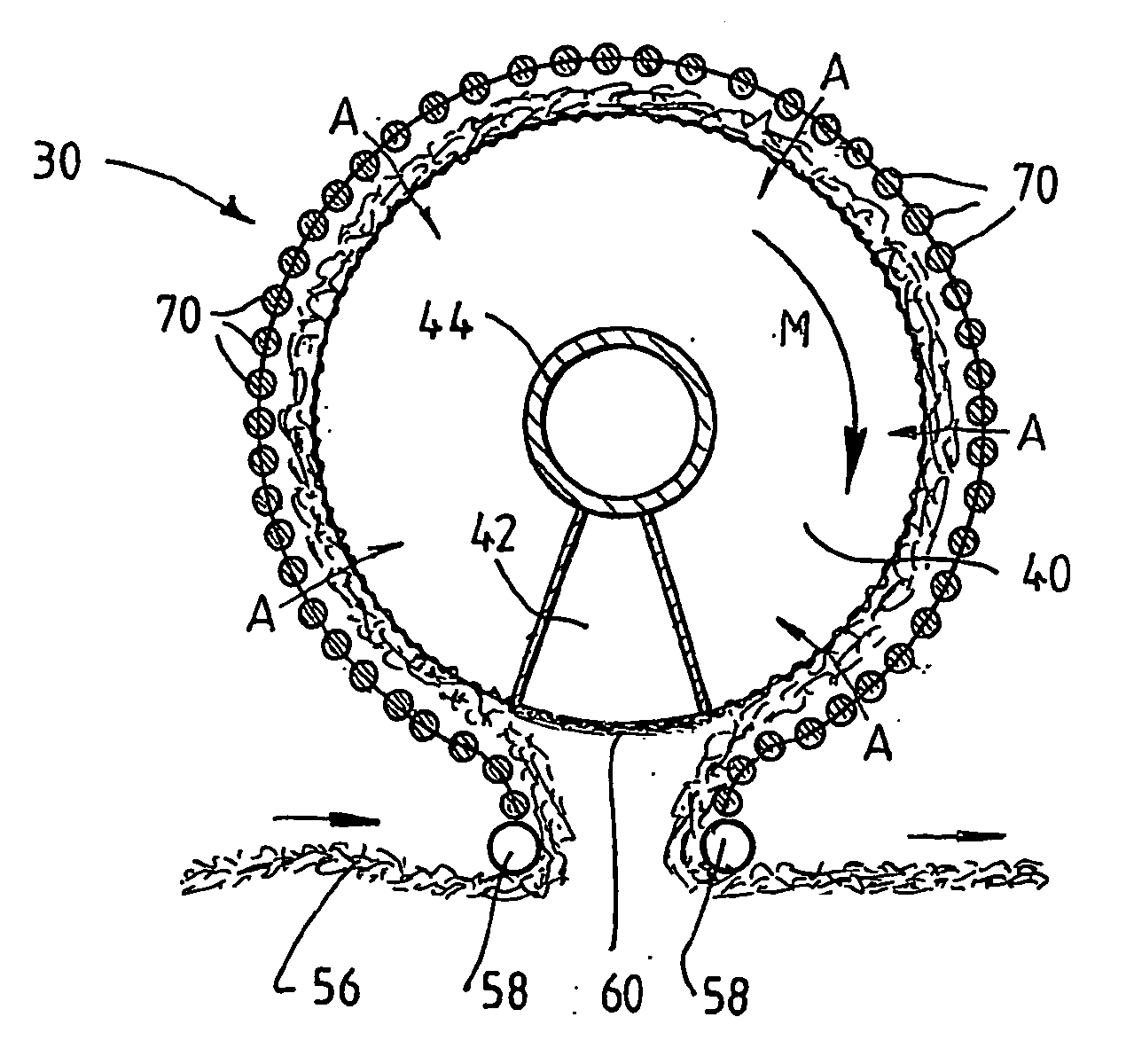
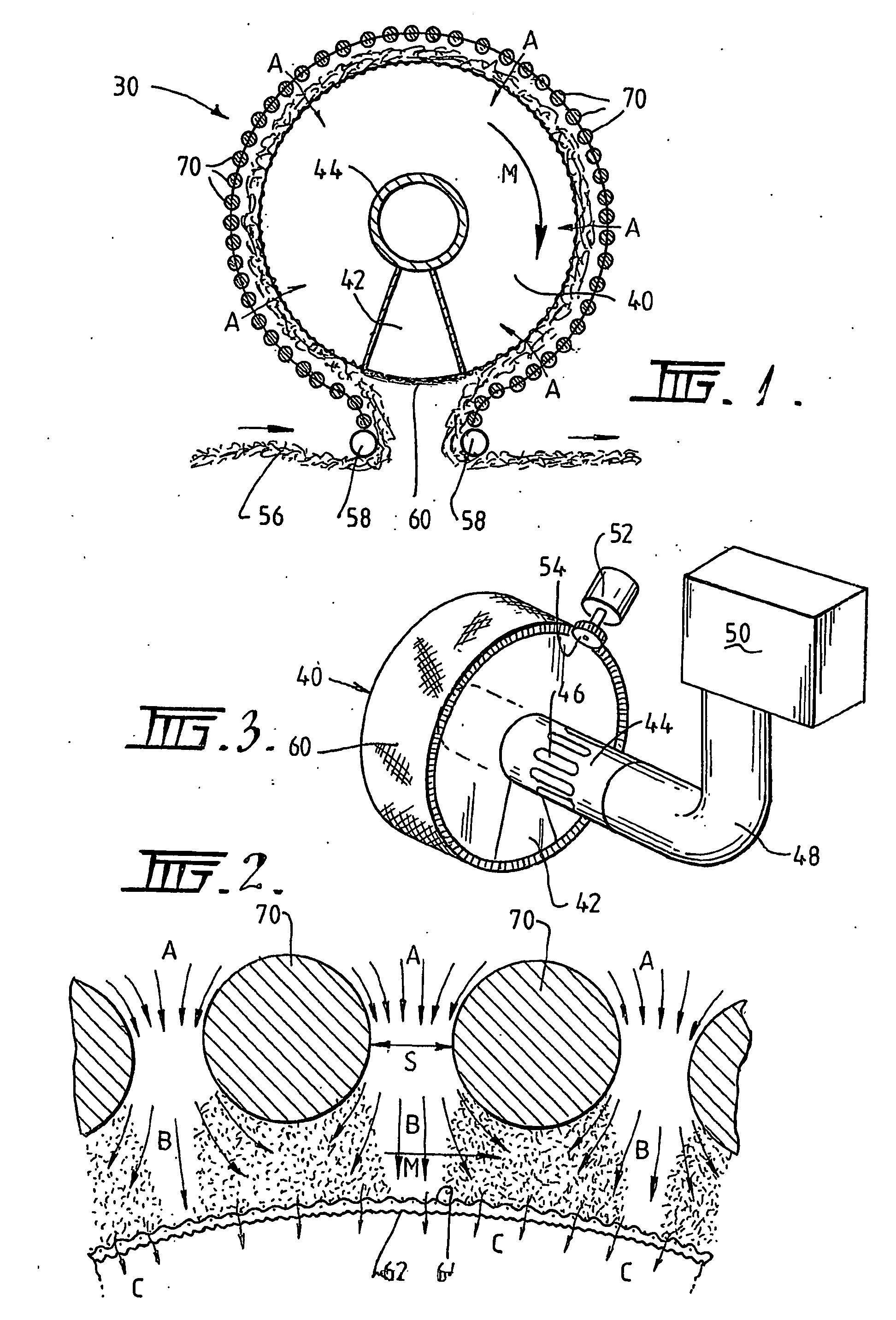
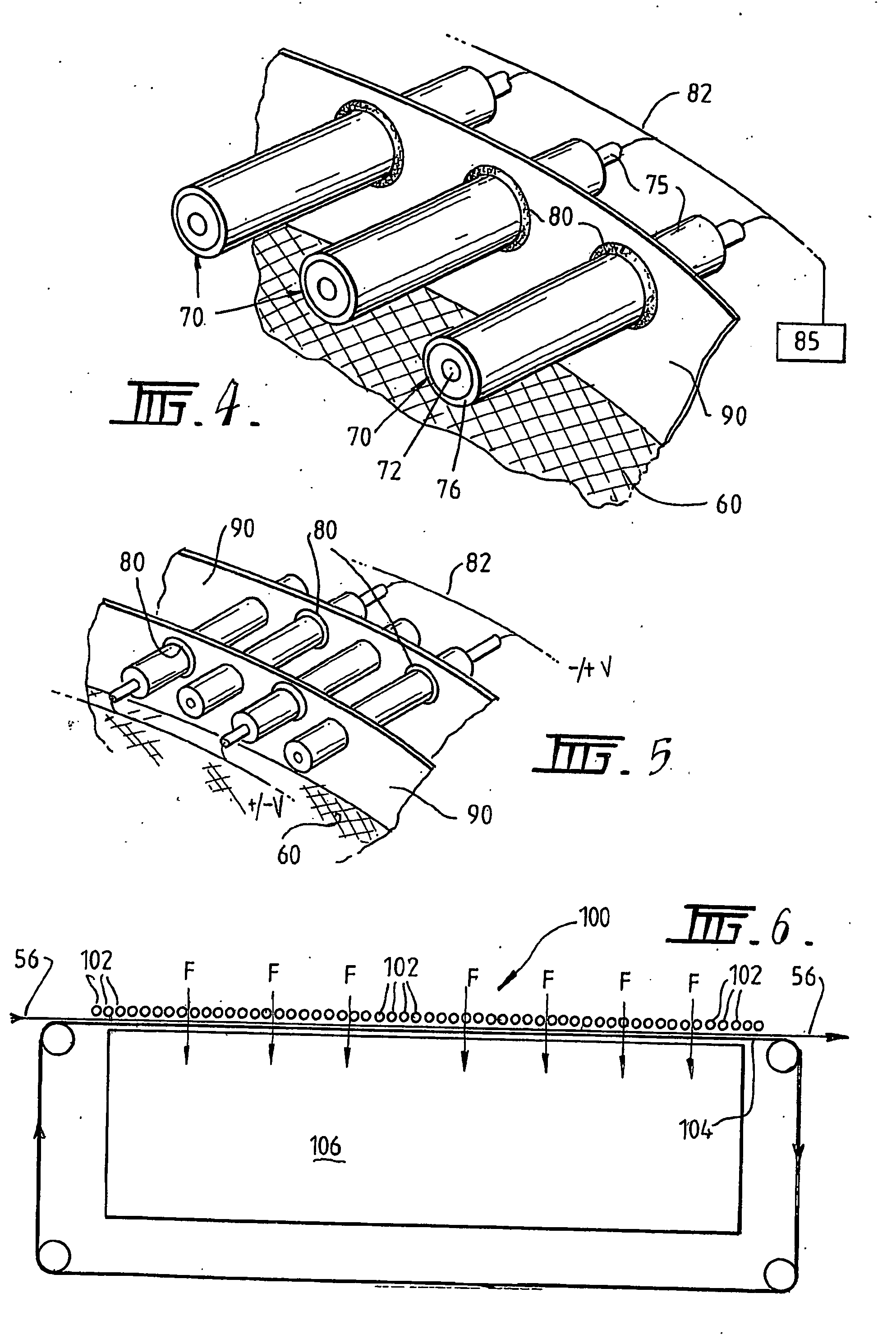
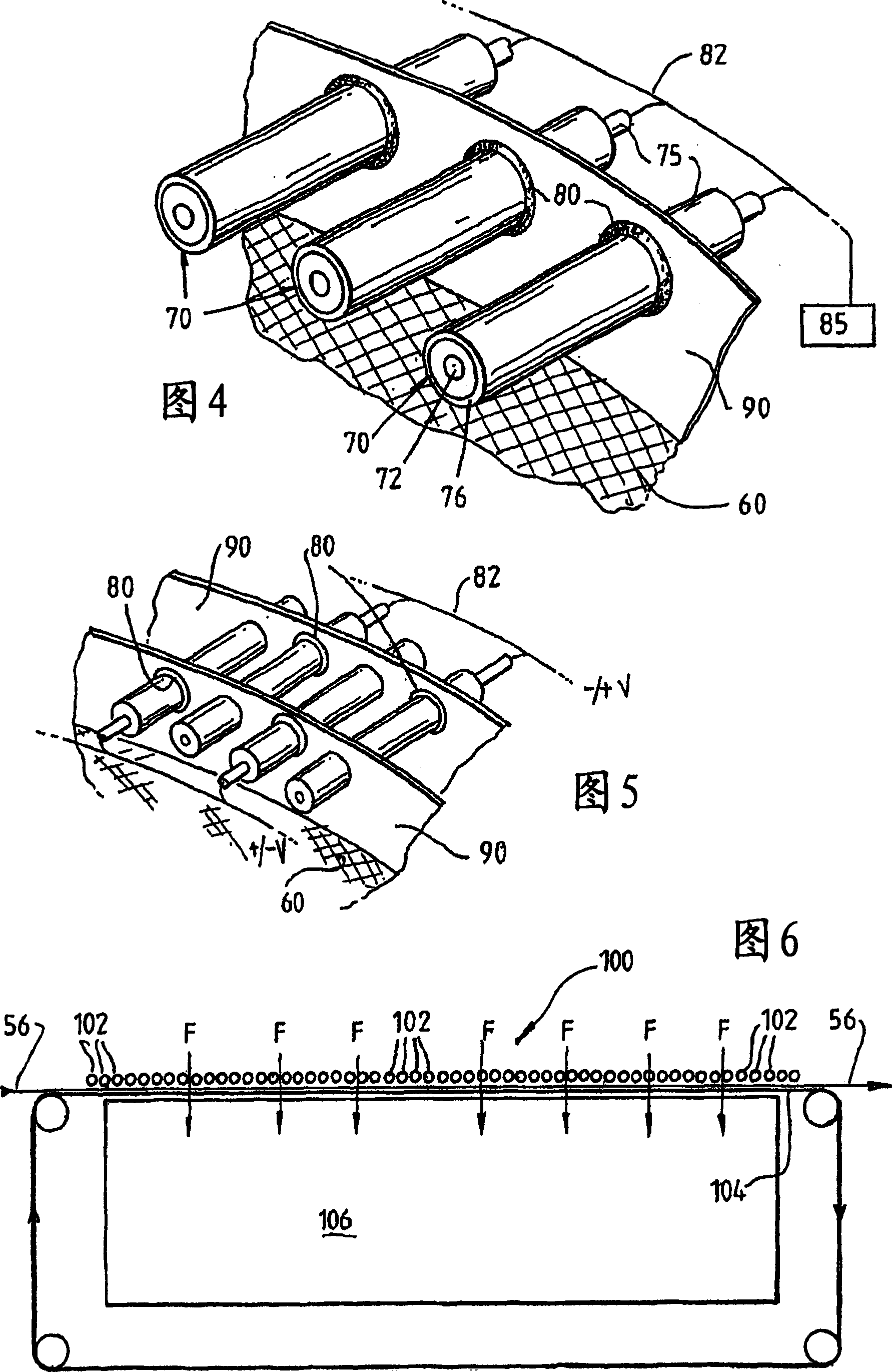
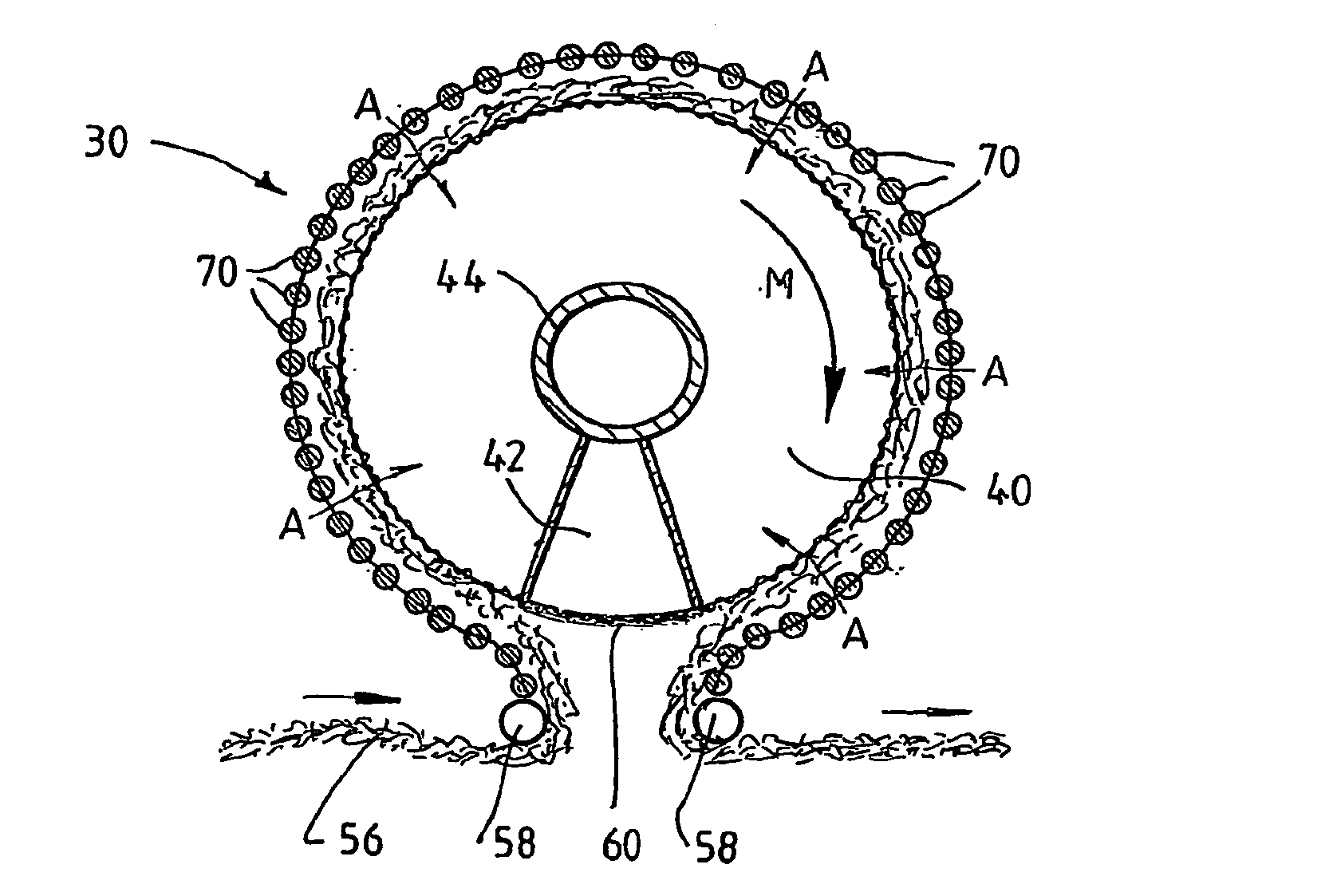
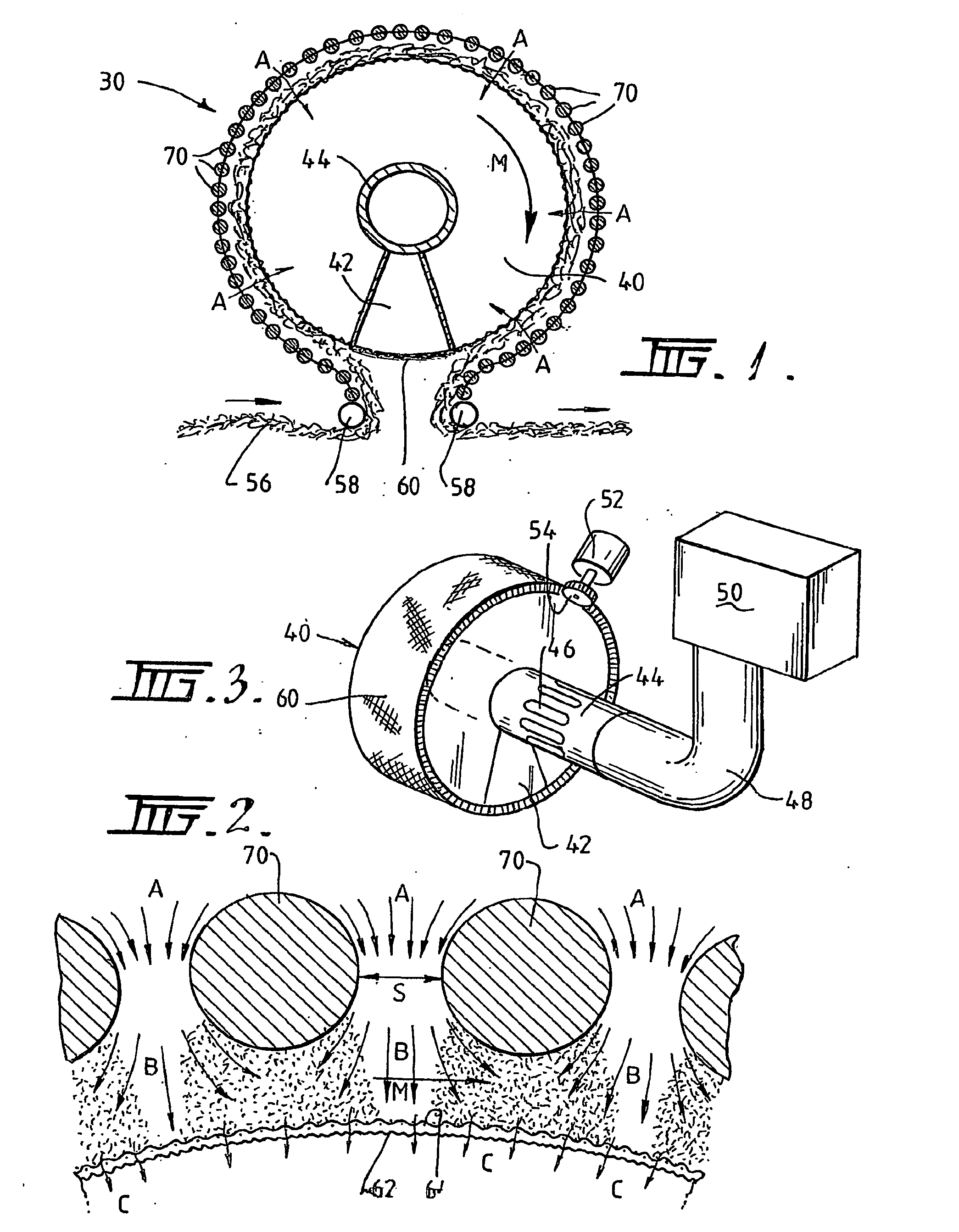
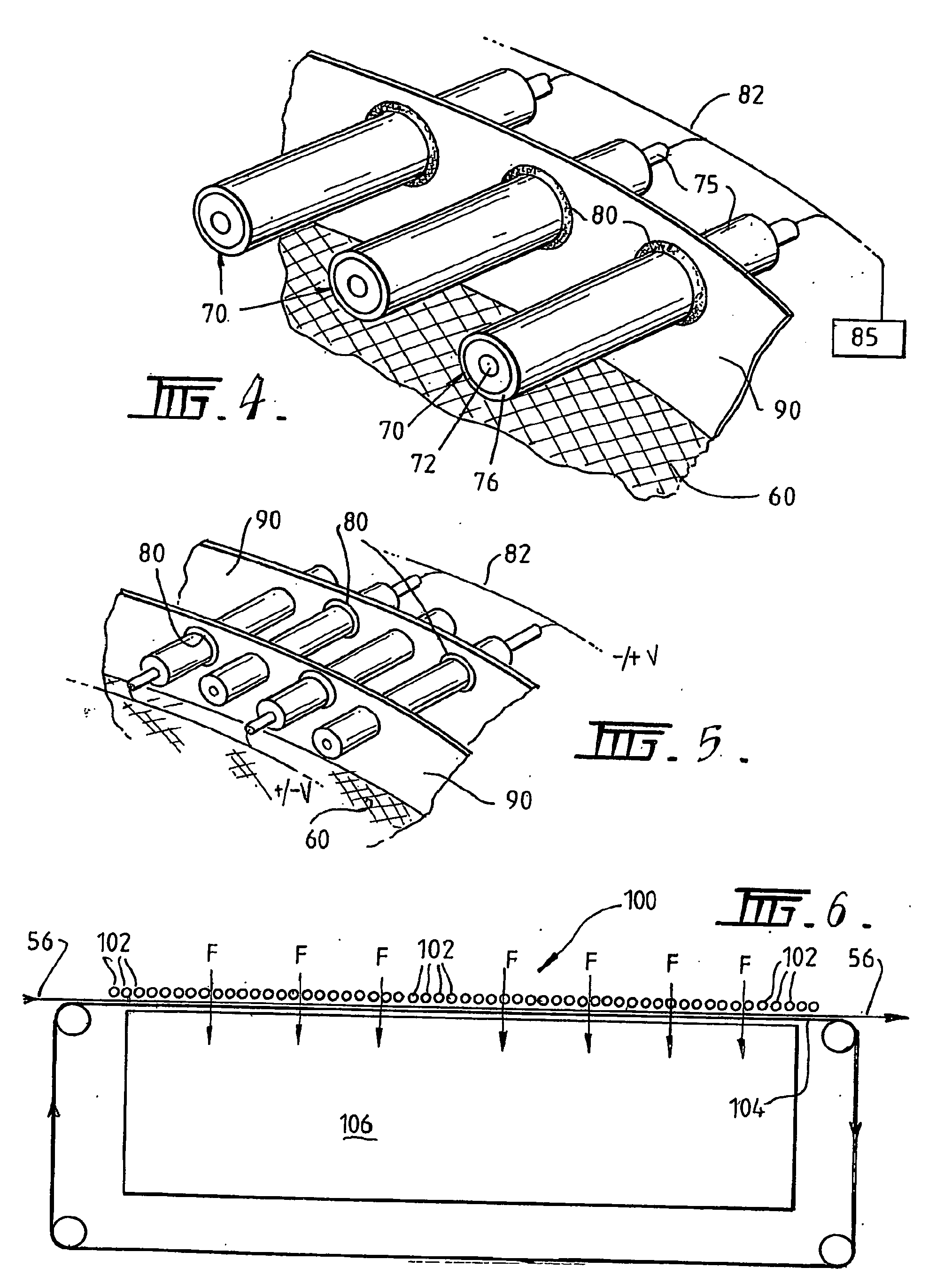
A plasma treated gas permeable material is produced by applying an alternating voltage between spaced electrodes, at least one of which is covered with a dielectric barrier and at least one of which comprises a plurality of discrete electrode segments, to generate plasma microdischarges between the spaced electrodes. A gas permeable material is passed between or adjacent to the spaced electrodes. A gas is moved between the electrode segments into and through the space between the electrodes and through the gas permeable material. The gas flows over plasma generation surfaces of the respective electrode segments and is moved at a rate whereby the gas flow between the spaced electrodes is turbulent and so randomises the plasma microdischarges and disperses plasma products that would otherwise give rise to burning instabilities in the gas permeable material, whereby the randomized plasma microdischarges provide a generally uniform plasma treatment of the gas permeable material. Also disclosed is an apparatus for laying out the process.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Surface preparation prior to deposition
InactiveUS7056835B2Without significantly affectingNucleation is easySemiconductor/solid-state device manufacturingChemical vapor deposition coatingGate dielectricAtomic layer deposition
Methods are provided herein for treating substrate surfaces in preparation for subsequent nucleation-sensitive depositions (e.g., polysilicon or poly-SiGe) and adsorption-driven deposition (e.g. atomic layer deposition or ALD). Prior to depositing, the surface is treated with non-depositing plasma products. The treated surface more readily nucleates polysilicon and poly-SiGe (such as for a gate electrode), or more readily adsorbs ALD reactants (such as for a gate dielectric). The surface treatment provides surface moieties more readily susceptible to a subsequent deposition reaction, or more readily susceptible to further surface treatment prior to deposition. By changing the surface termination of the substrate with a low temperature radical treatment, subsequent deposition is advantageously facilitated without depositing a layer of any appreciable thickness and without significantly affecting the bulk properties of the underlying material. Preferably less than 10 Å of the bulk material incorporates the excited species, which can include fluorine, chlorine and particularly nitrogen excited species.
Owner:ASM IP HLDG BV
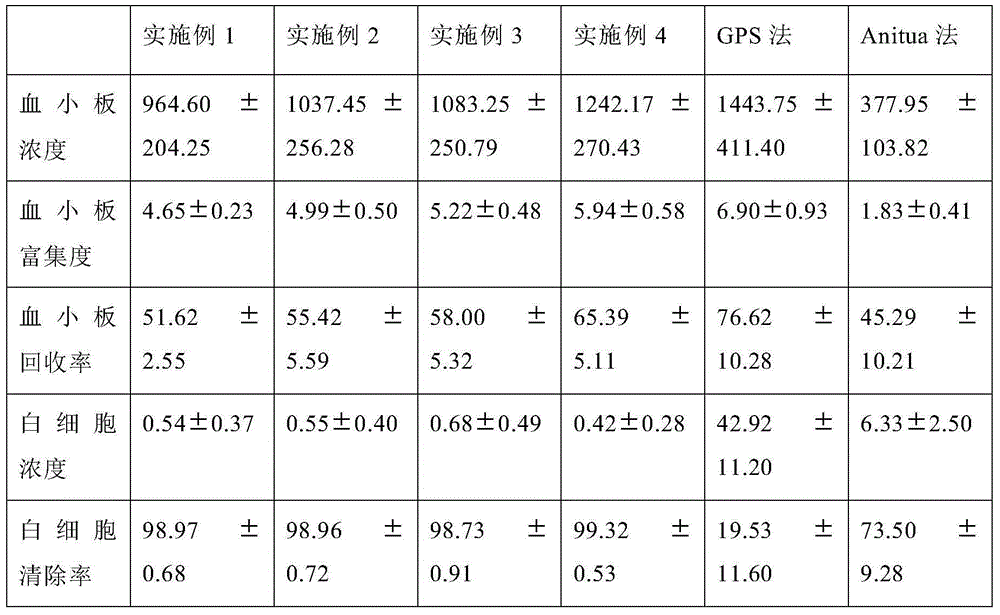
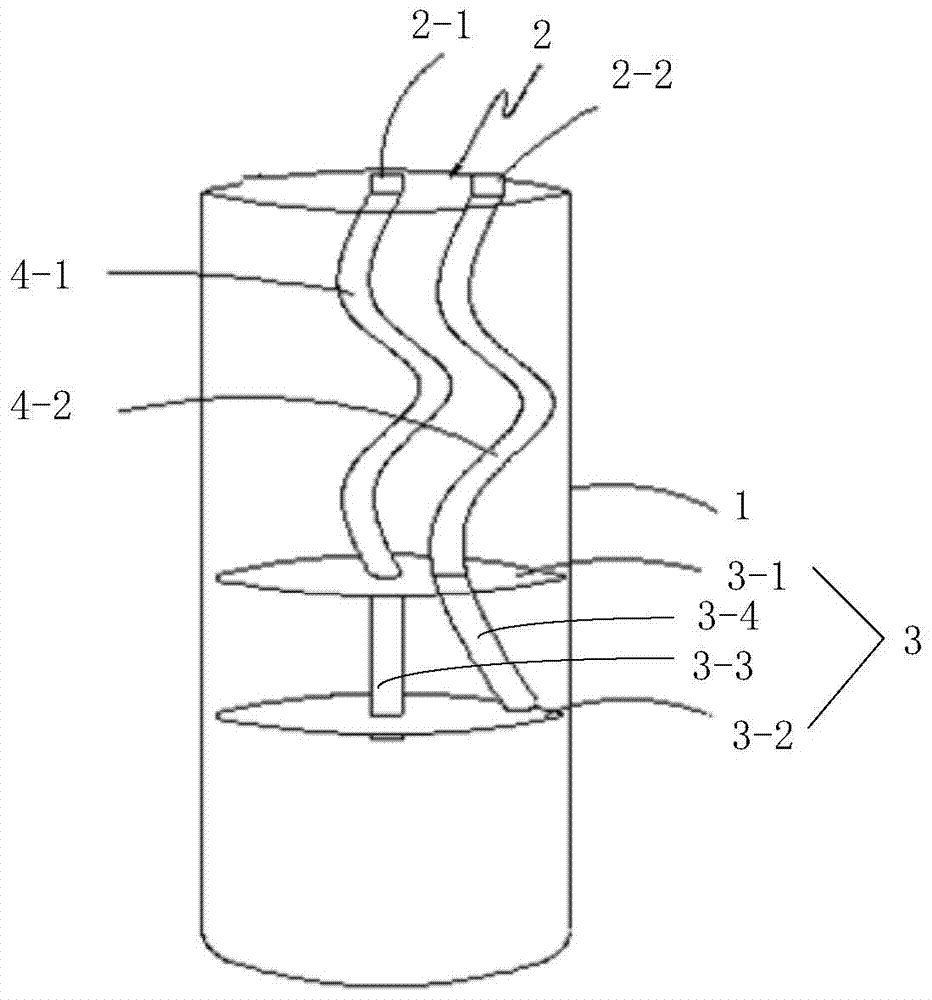
Preparation method and device for leukocyte-depleted platelet rich plasma
InactiveCN105107233ALow incidence of adverse reactionsEasy to operateCentrifugesLiquid separationWhole blood productWhite blood cell
The invention belongs to the blood product technology field, and discloses a preparation method for leukocyte-depleted platelet rich plasma. The method comprises the following steps: firstly, anticoagulants and blood are drawn and mixed, and whole blood to be separated is obtained; secondly, first centrifugation is carried out, a centrifugate divided into three layers from bottom to top is obtained, and platelet and plasma components in the upper layer are obtained; thirdly, the obtained upper layer components are centrifuged again, and platelet deposition at the bottom and a plasma supernatant are formed; part of the plasma supernatant is drawn, the platelet deposition is subjected to resuspension by the left plasma supernatant, and leukocyte-depleted platelet rich plasma is prepared. The method achieves elimination of leukocytes and enrichment of platelets at the same time, operation is convenient, consumed time is short, the technical indexes of the prepared plasma product are better than technical indexes of present products with the same kind. Centrifugation for two times in the method can be carried out in two centrifuge tubes respectively, or be carried out in one centrifugal device. The invention provides a centrifugal device used for performing the preparation method.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Plasma sorting system and method
The invention belongs to the technical field of plasma products, and discloses a plasma sorting system and method. The plasma sorting system is characterized in that plasma bottles are grabbed and moved by a manipulator, reinspection results of current products are acquired by use of an identification unit and a controller, the corresponding plasma bottles are placed in a classified manner according to the acquired results, and qualified products are separated from unqualified products; and a loading line is used for automatic loading, an unloading line is used for automatic unloading, the whole process is completely operated by the manipulator, and the identification process does not need manual participation, so that the phenomenon of sorting mistakes is little. The labor intensity of plasma sorting is reduced, and the sorting working efficiency and the quality of blood products are improved.
Owner:北京达特集成技术有限责任公司
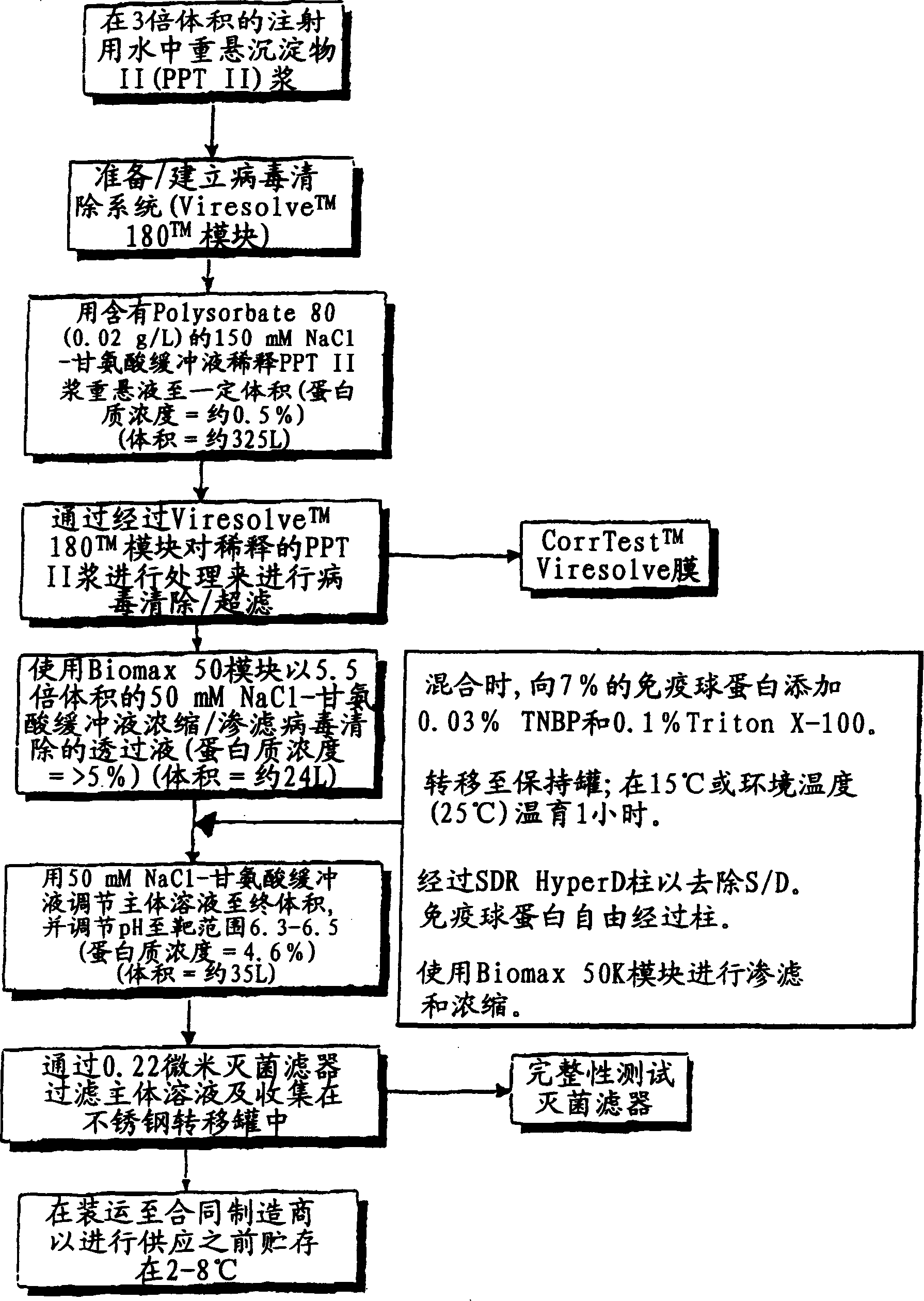
Viral reduction method for plasma using a leukocyte-reduction filter and two virus-reduction filters of decreasing pore diameters
ActiveUS7592134B2Efficient methodEfficient removalOther blood circulation devicesHaemofiltrationWhite blood cellLeukocyte Reduction Filtration
The present invention relates to a plasma product or a serum product with an extremely low risk of viral contamination and a method for producing the same. Before treating plasma or serum to be used as a raw material for producing a plasma product or a serum product using a virus removal membrane, leucocytes contaminating the blood are removed. Thus, a plasma product or a serum product with an extremely low risk of viral contamination can be efficiently produced while preventing clogging. Since clogging scarcely arises, it is possible to carry out efficient filtration without applying an elevated pressure as the filtration proceeds.
Owner:ASAHI KASEI MEDICAL CO LTD
Blood plasma product for clinical transfusion and preparation method thereof
The invention discloses a blood plasma product for clinical transfusion and a preparation method thereof. The blood plasma product is prepared from cord blood. The preparation method is as follows: the cord blood in placeta umbilic, which is wasted from a term birth infant, is collected and is centrifuged, and nucleated cells are removed; ABO and Rh blood type detection, blood cell and blood plasma component measurement, pathogenic microorganism immunoassay and germiculture are carried out on the obtained blood plasma without the nucleated cells; and qualified blood plasma is detected, a proper amount of protective agent is added, spin-freeze is carried out at the temperature of minus 35 DEG C for 2 hours, and then freeze-drying is carried out at the temperature of 35 DEG C-37 DEG C within 18-20 hours to obtain the freeze-dried cord blood plasma product. The transfusion comprises common clinical transfusion and transfusion in the aspects of malignant tumor subject to chemo-treatment / radiation treatment and poor hematopoiesis diseases caused by aplastic anemia. The cord blood is a special transfusion resource with important treatment function and also a source of important candidate stem cells.
Owner:UNION STEMCELL & GENE ENG
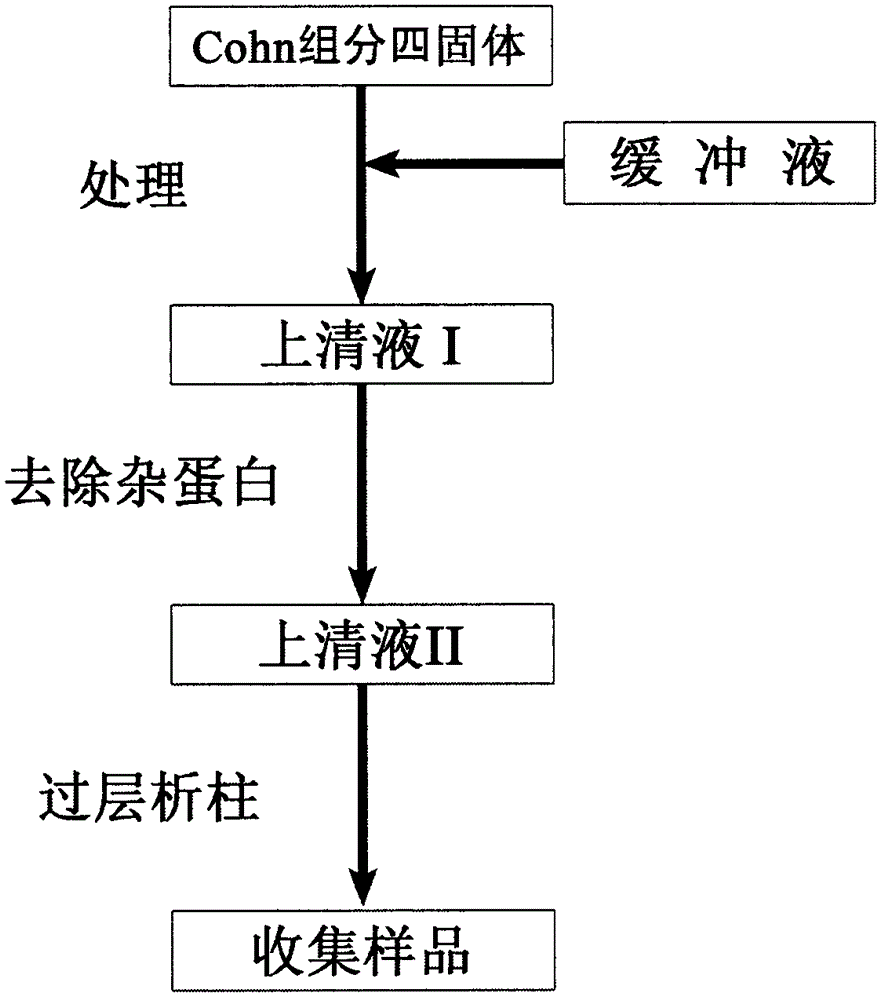
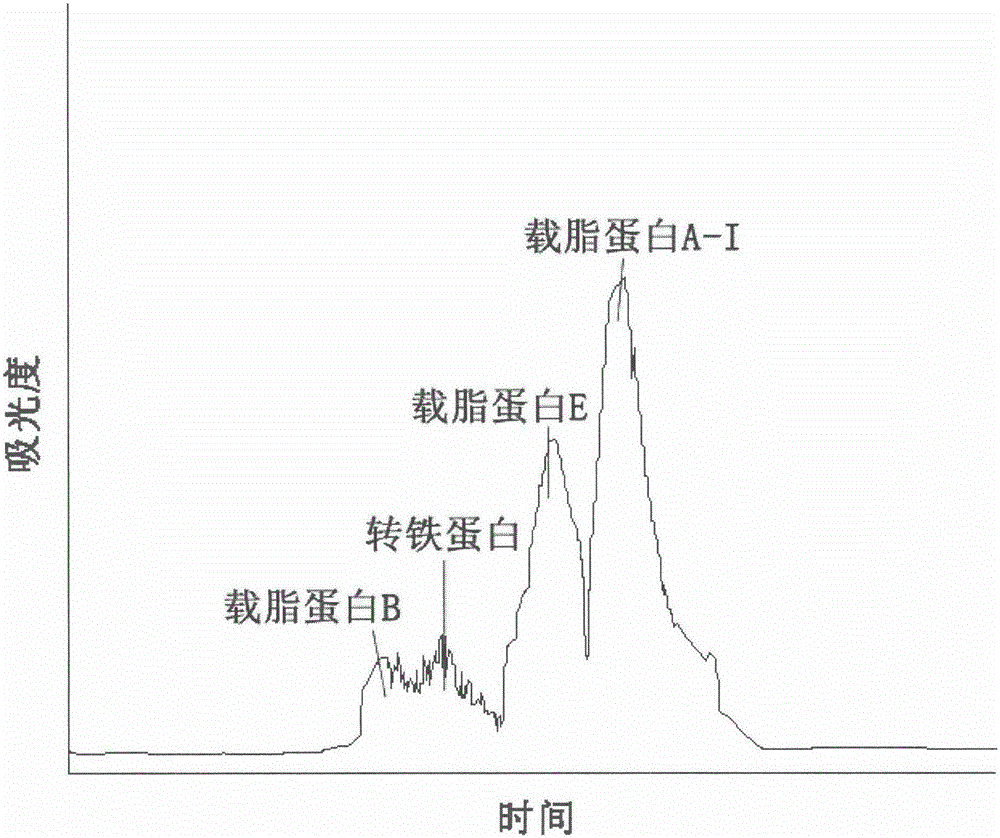
Method for purifying plasma functional proteins from Cohn fraction IV
InactiveCN106279405AHigh purityHigh activityNervous disorderPeptide/protein ingredientsFreeze-dryingPre treatment
The invention discloses a method for purifying plasma functional proteins from a Cohn fraction IV. The method comprises the following steps: carrying out pretreatment and removing a filter aid and other proteins in the raw materials; carrying out gradient separation by chromatography and collecting the plasma functional proteins in sequence; carrying out sterilization and freeze-drying. The method is high in protein yield and low in cost, comprises the purification process with the pipeline characteristic, has high degree of automation, is suitable for industrialization and achieves comprehensive utilization of the byproduct in plasma product production.
Owner:CHINA PHARM UNIV
Plasma treatment apparatus and method
InactiveCN1784519AWon't burnUniform treatmentUltrasonic/sonic fibre treatmentEnergy based chemical/physical/physico-chemical processesCombustion instabilityCombustion
Plasma-treated gas-permeable material produced by applying an alternating voltage between spaced apart electrodes, at least one of which is coated with a dielectric barrier layer, at least one of which consists of a plurality of separate electrode segments , thereby generating plasma microelectrodes between the separated electrodes. A gas permeable material is passed between or adjacent to the spaced electrodes. Gas moves between the electrode segments into and through the spaces between the electrodes and through the gas permeable material. The gas flows across the plasma-generating surfaces of the corresponding electrode segments at a rate such that the gas flow between the electrodes thus separated is in a turbulent state and causes the plasma micro-emissions to randomize and disperse the plasma products, These products would otherwise cause combustion instabilities in the gas permeable material, so that the randomized plasma microdischarge can provide an overall uniform plasma treatment of the gas permeable material. At the same time, the equipment used for the design process is also disclosed.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Dry etching method
InactiveUS20070000868A1Decorative surface effectsSemiconductor/solid-state device manufacturingHydrogenFluorocarbon
A dry etching method comprises: exposing an etching region of a workpiece to a plasma product of a depositive gas, the depositive gas having a CF group in a reaction chamber; exposing the etching region to a plasma product of an etching gas in the reaction chamber; exposing the etching region to a plasma product of the depositive gas in the reaction chamber; and exposing the etching region to a plasma product of the etching gas in the reaction chamber. Alternatively, a dry etching method comprises: exposing an etching region of a workpiece to a plasma product of a depositive gas, the depositive gas containing a fluorocarbon-based gas and at least one of CO (carbon monoxide) gas, hydrogen gas, and CH4 gas in a reaction chamber; exposing the etching region to a plasma product of an etching gas in the reaction chamber; exposing the etching region to a plasma product of the depositive gas in the reaction chamber; and exposing the etching region to a plasma product of the etching gas in the reaction chamber.
Owner:KK TOSHIBA
Plasma treatment apparatus and method
InactiveUS20110180387A1Increase spawn rateIncrease temperatureUltrasonic/sonic fibre treatmentEnergy based chemical/physical/physico-chemical processesCombustion instabilityElectricity
A plasma treated gas permeable material is produced by applying an alternating voltage between spaced electrodes, at least one of which is covered with a dielectric barrier and at least one of which comprises a plurality of discrete electrode segments, to generate plasma microdischarges between the spaced electrodes. A gas permeable material is passed between or adjacent to the spaced electrodes. A gas is moved between the electrode segments into and through the space between the electrodes and through the gas permeable material. The gas flows over plasma generation surfaces of the respective electrode segments and is moved at a rate whereby the gas flow between the spaced electrodes is turbulent and so randomises the plasma microdischarges and disperses plasma products that would otherwise give rise to burning instabilities in the gas permeable material, whereby the randomized plasma microdischarges provide a generally uniform plasma treatment of the gas permeable material. Also disclosed is an apparatus for laying out the process.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Reflective mask cleaning apparatus and reflective mask cleaning method
InactiveUS20170017151A1Suppresses deterioration of optical propertiesPhotomechanical apparatusOriginals for photomechanical treatmentOrganic solventFree solution
A reflective mask cleaning apparatus according to an embodiment comprises a first supply section configured to supply a first solution containing at least one of an organic solvent and a surfactant to a ruthenium-containing capping layer provided in a reflective mask; and a second supply section configured to supply at least one of a reducing solution and an oxygen-free solution to the capping layer.A reflective mask cleaning apparatus according to an alternative embodiment comprises a third supply section configured to supply a plasma product produced from a reducing gas to a ruthenium-containing capping layer provided in a reflective mask; and a second supply section configured to supply at least one of a reducing solution and an oxygen-free solution to the capping layer.
Owner:SHIBAURA MECHATRONICS CORP
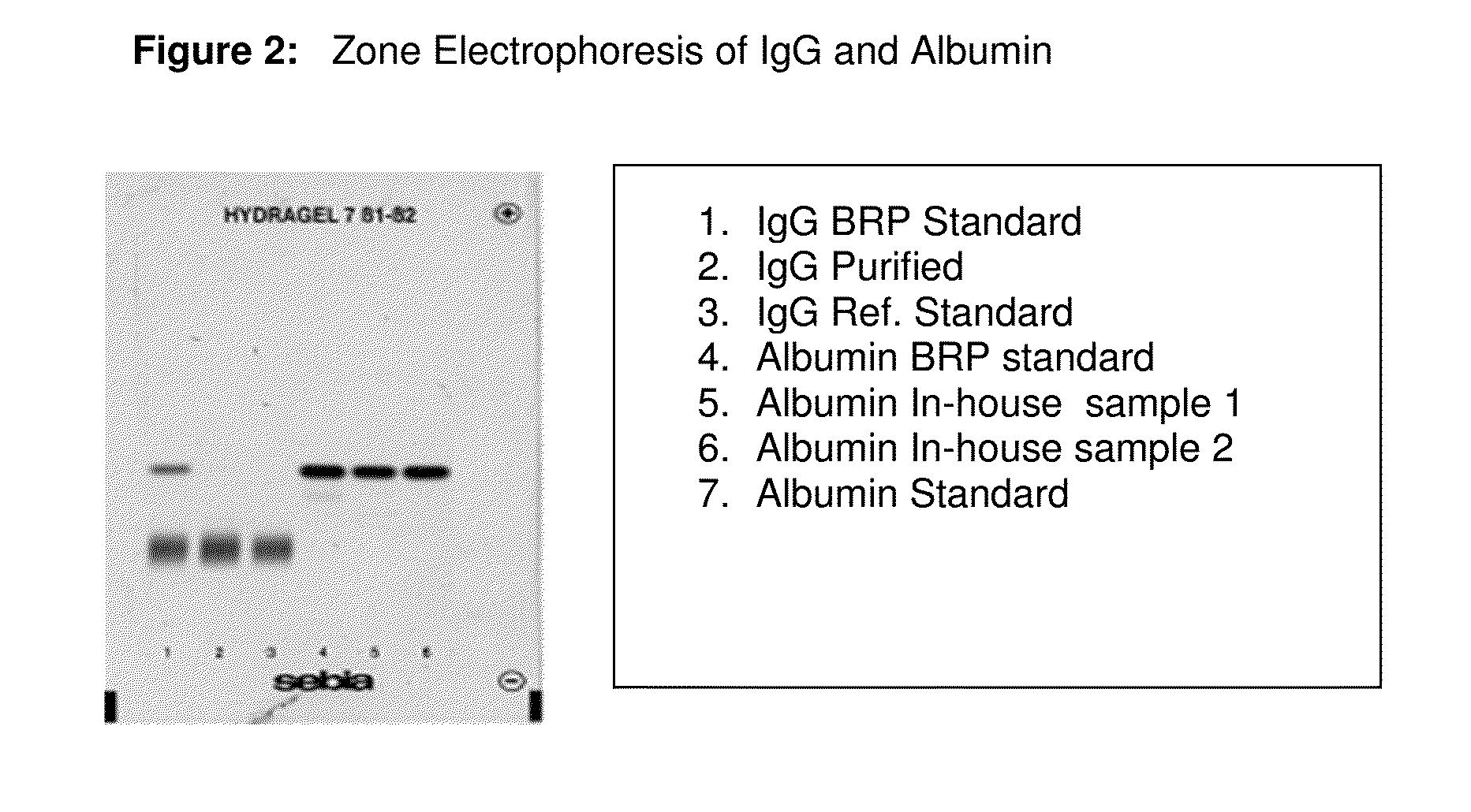
Integrated process for the production of therapeutics (human albumin, intravenous immunoglobulins, clotting factor viii and clotting factor ix) from human plasma
ActiveUS20150210737A1Low costImprove affordabilityHydrolasesChemical industryFractionationHuman albumin
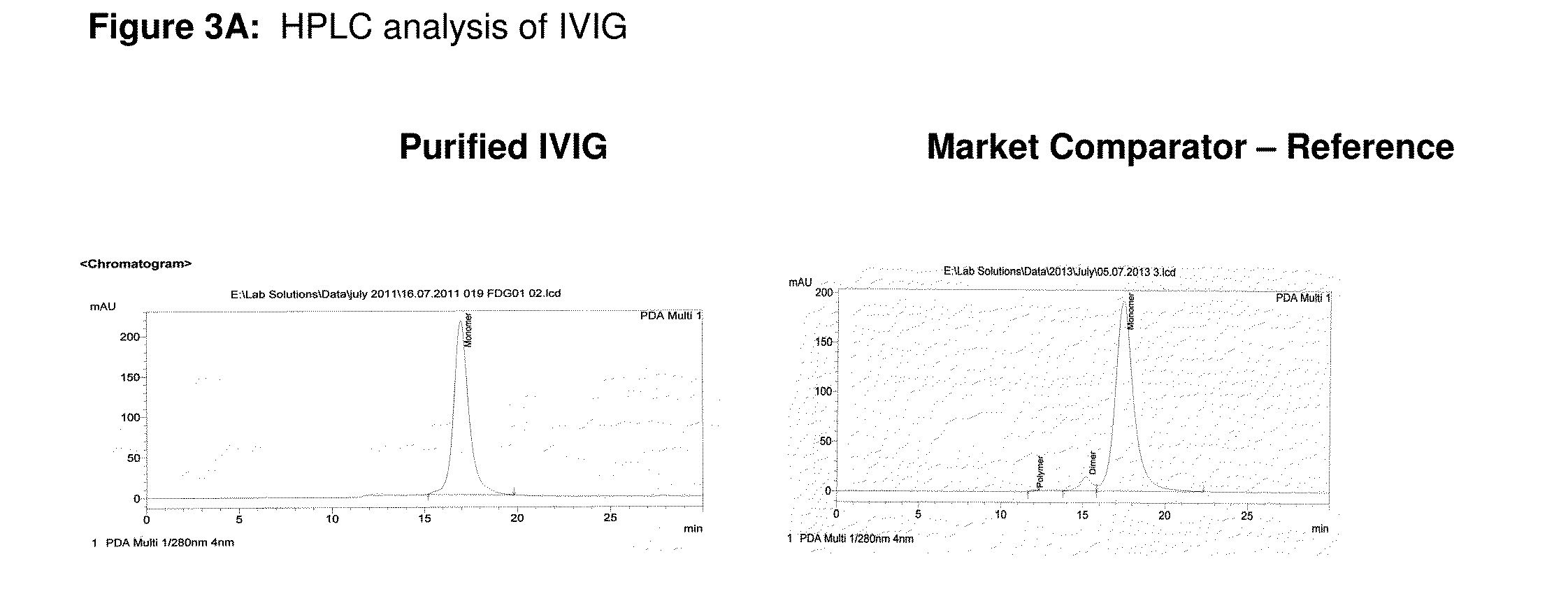
The invention relates to an integrated scheme for fractionation and purification of plasma products (human albumin, intravenous immunoglobulin (IVIG), clotting factor VIII and clotting factor IX) by sequential chromatography and virus reduction steps. The therapeutically administrable protein IVIG has purity levels exceeding 98%, aggregates and dimers at less than 0.2%, Fc function of >90% and anti-complementary activity of less than 0.5 CH50 per mg of Ig. The distribution of IgG isomers is comparable to the ranges seen in normal plasma. Human albumin for therapeutic use, purified by this integrated scheme has an electrophoretic purity of close to 100%, with monomers exceeding 98%. The levels of aluminium and pre-kallikrein activator are below the detection limit for the respective tests. The Factor IX preparations have a specific activity of ≧200 IU / mg. The impurity levels of Factor-II, Factor VII, Factor X are at least 10-fold lesser (≦0.5% instead of 5%) and the heparin impurity of ≦0.01 IU (against 0.5 IU limit for this impurity) is 50-fold lesser the specified pharmacopoeial limits.The purification carried out by an all-chromatography scheme, avoids the use of ethanol precipitation in the entire manufacturing process of the said four plasma products. The invention describes an integrated process for purifying four different proteins from human plasma to high therapeutic grade purity levels, with a potential to purify more therapeutic proteins from a given plasma sample by incorporating additional chromatography steps in the sequence.
Owner:ICHOR BIOLOGICS PTE LTD
Materials and methods for blood plasma preparations
PendingUS20210069240A1Inorganic non-active ingredientsPharmaceutical containersBlood plasmaPharmacology
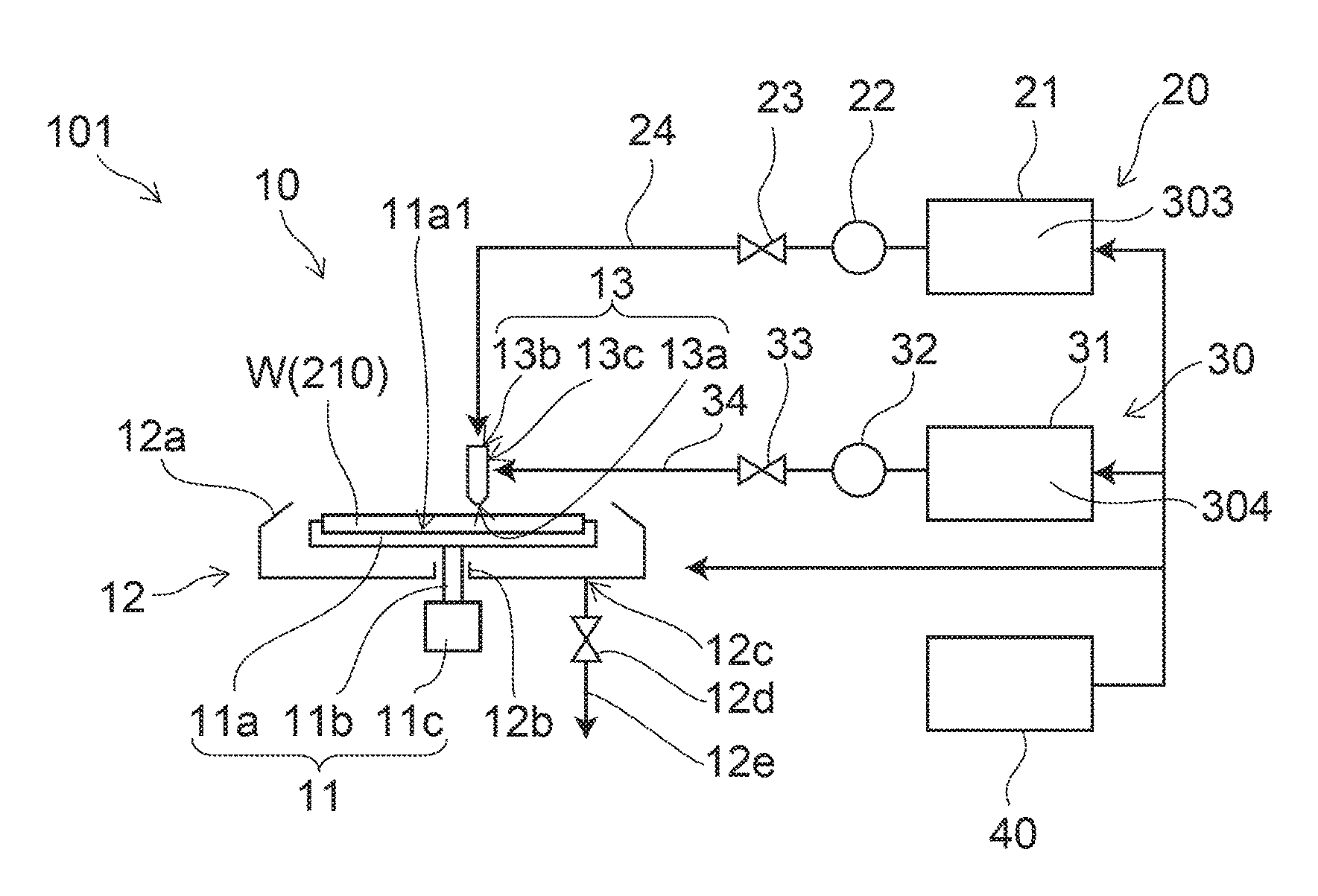
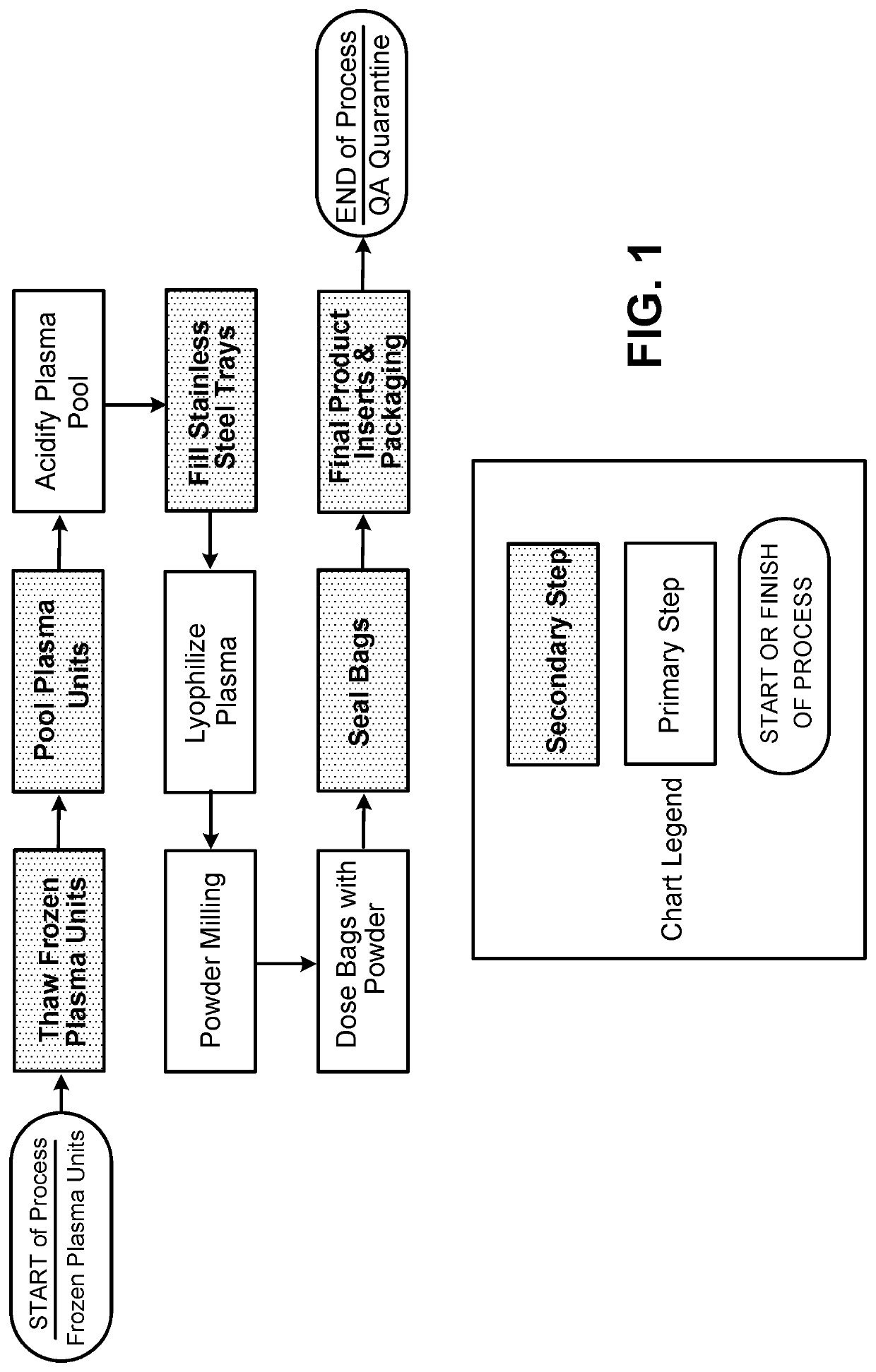
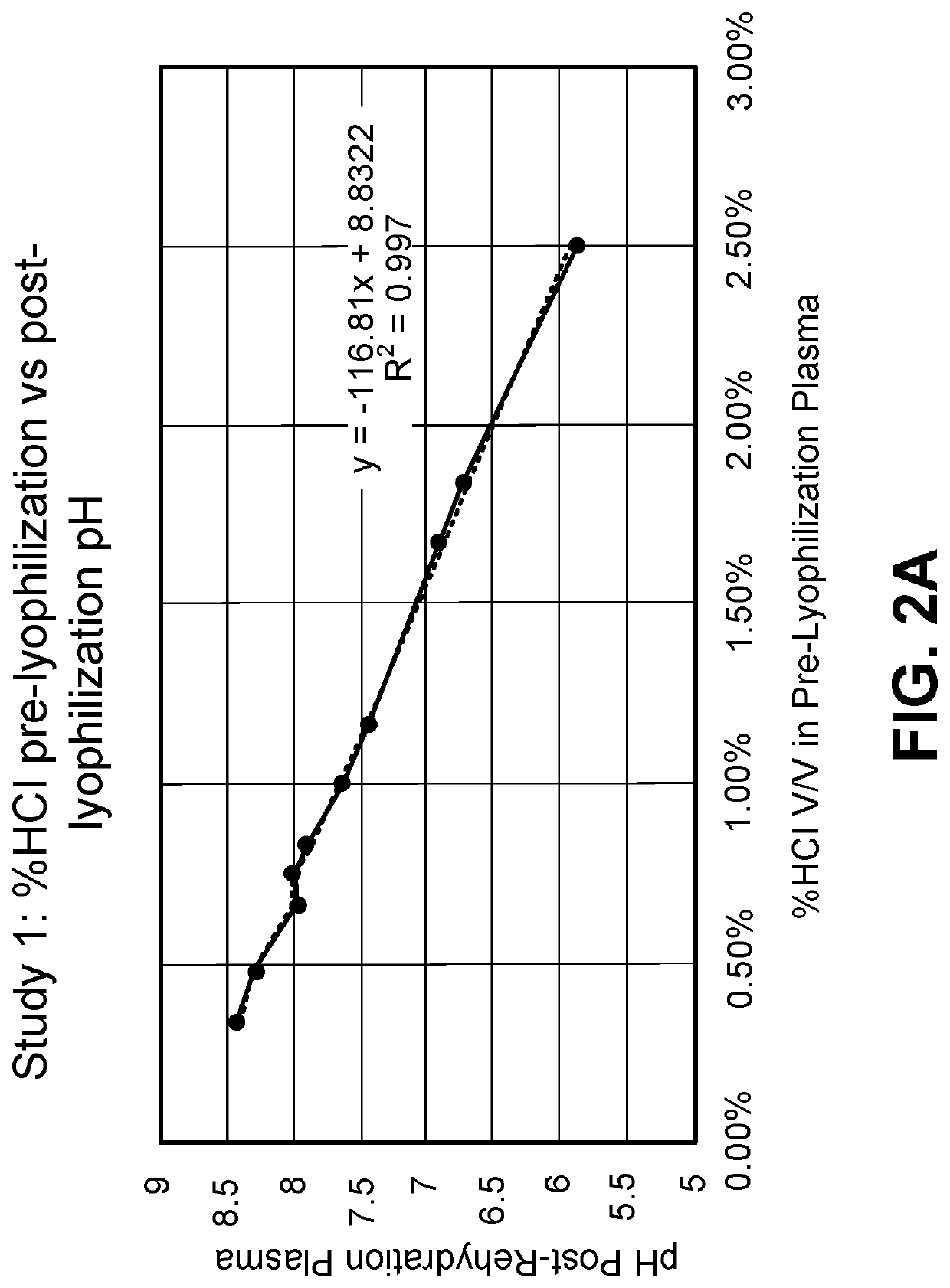
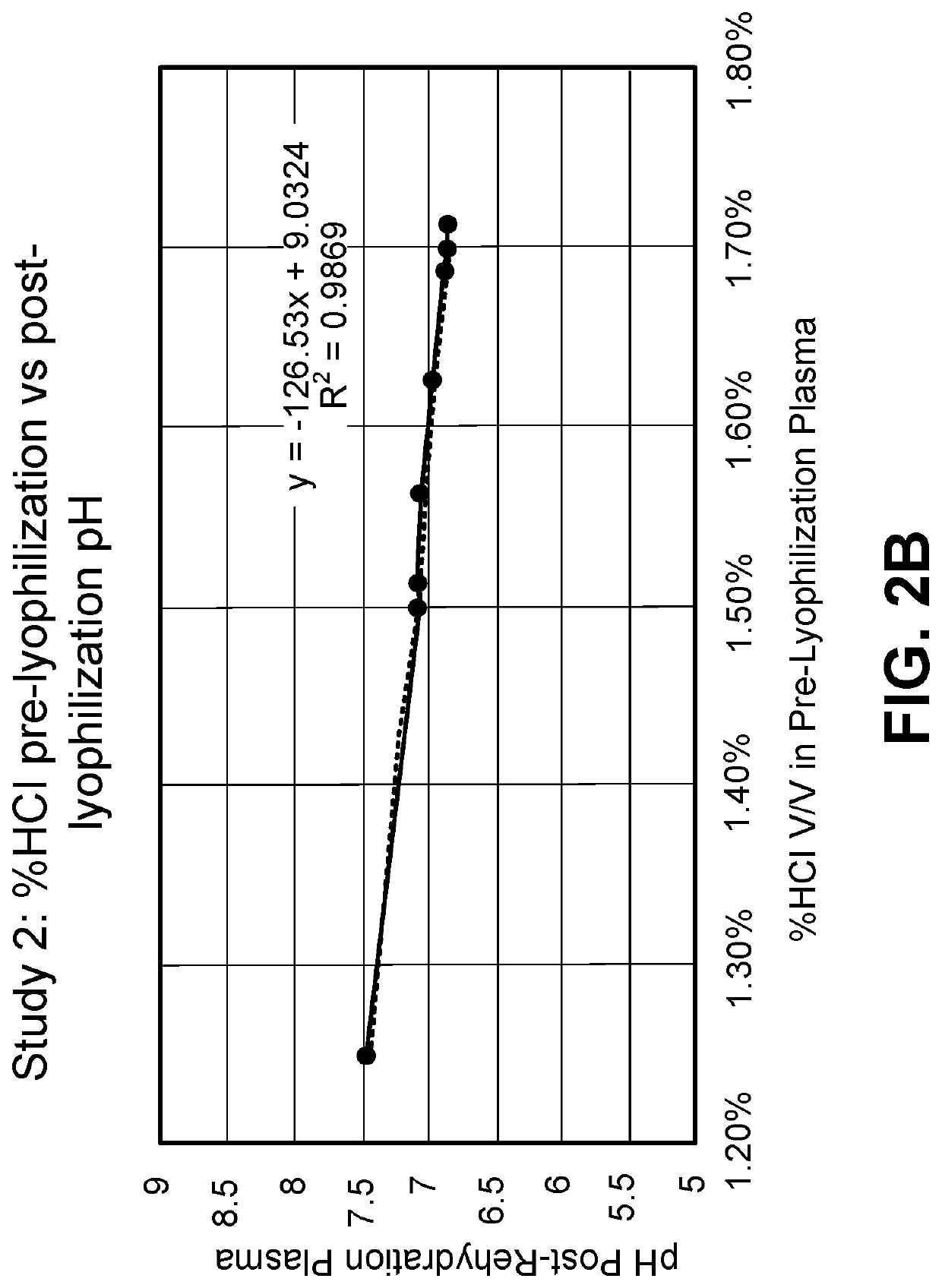
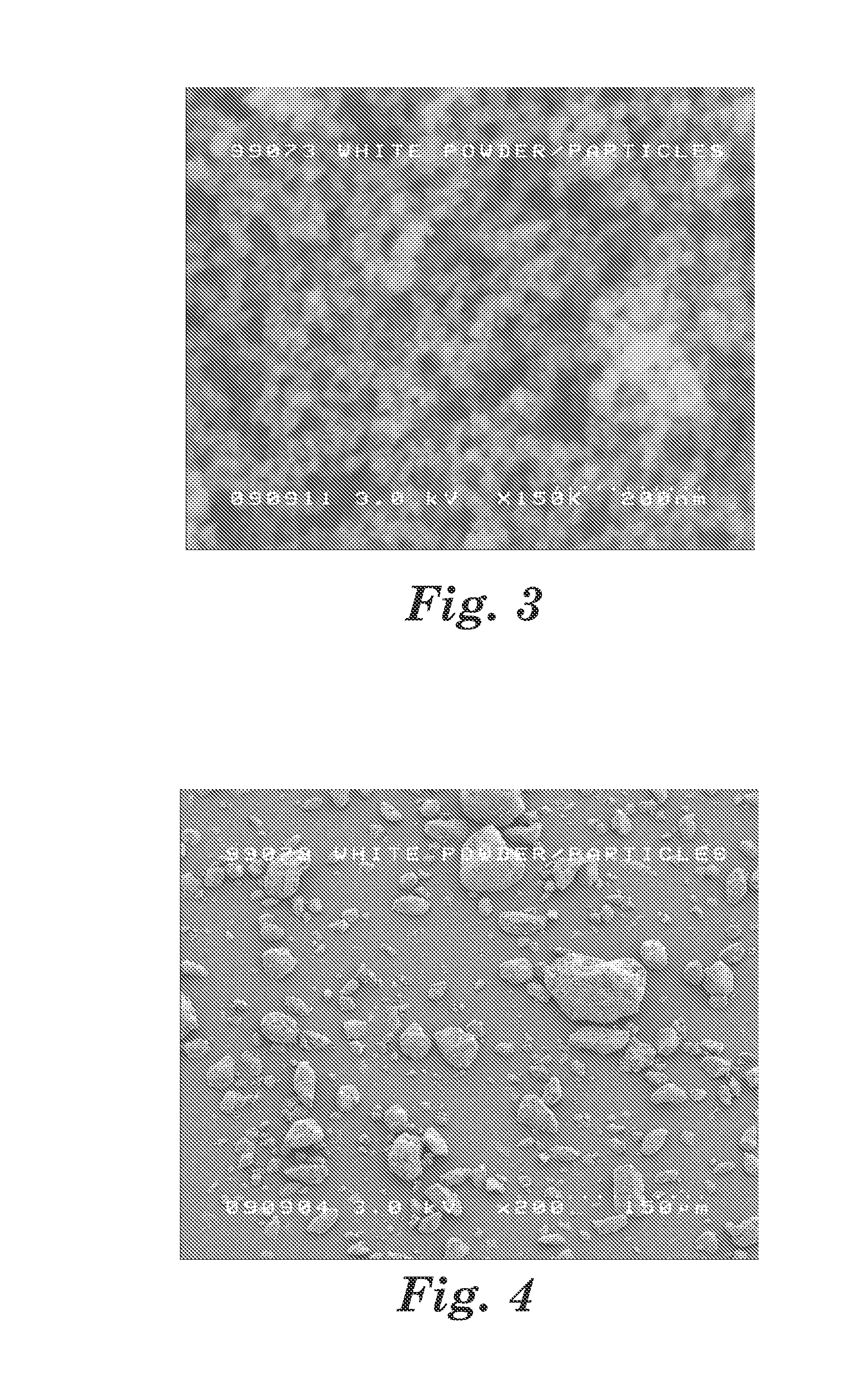
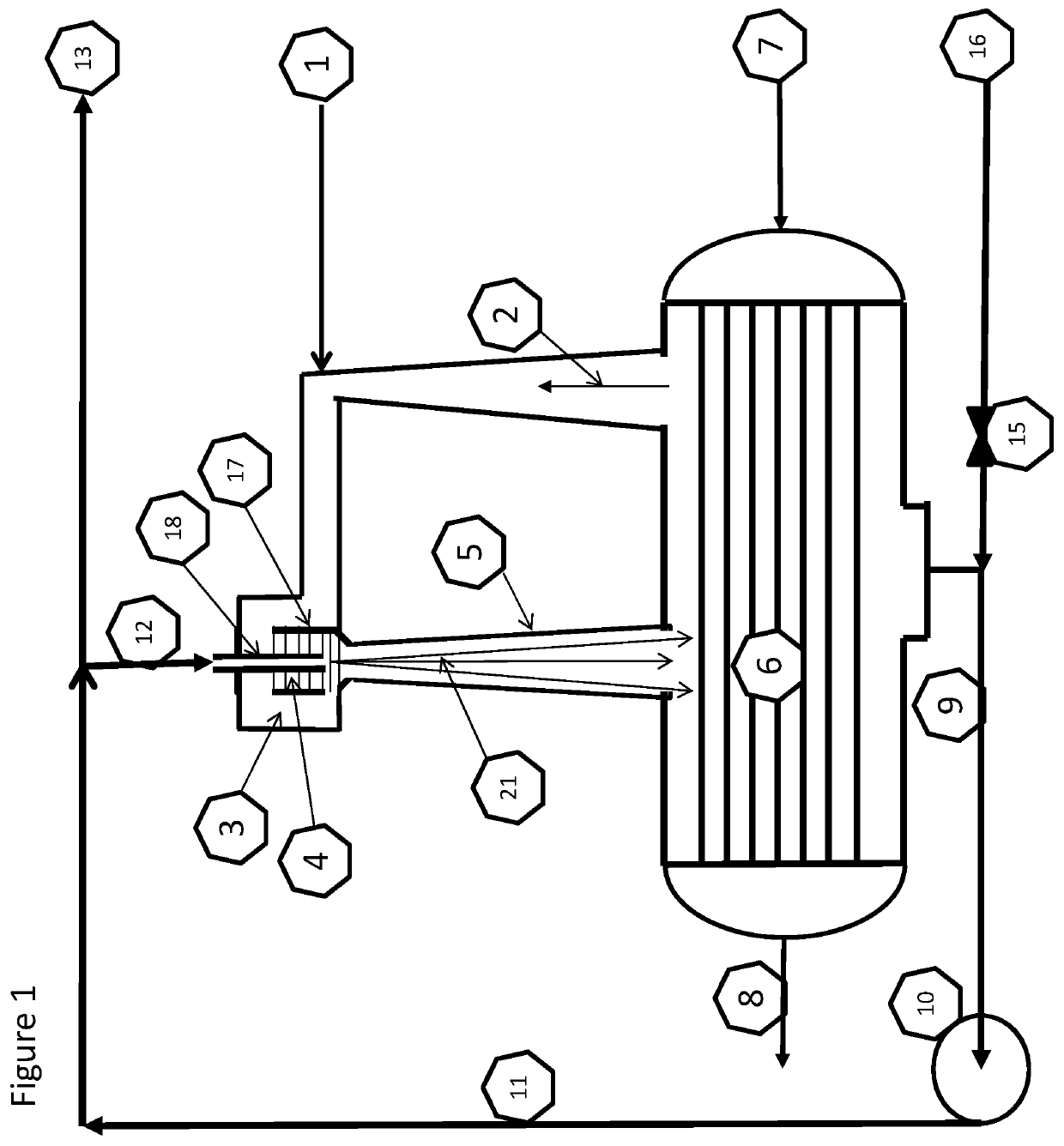
Provided herein in some embodiments is a method of preparing a plasma product, including providing plasma, acidifying the plasma to form acidified plasma, drying the acidified plasma to form dried plasma, and milling the dried plasma to form a plasma product. Also provided are plasma products produced by the methods described herein.
Owner:CELLPHIRE INC
Plasma preparation or serum preparation and process for producing the same
ActiveUS20060127874A1Efficient methodEfficient removalOther blood circulation devicesHaemofiltrationWhite blood cellBlood plasma
The present invention relates to a plasma product or a serum product with an extremely low risk of viral contamination and a method for producing the same. Before treating plasma or serum to be used as a raw material for producing a plasma product or a serum product using a virus removal membrane, leucocytes contaminating the blood are removed. Thus, a plasma product or a serum product with an extremely low risk of viral contamination can be efficiently produced while preventing clogging. Since clogging scarcely arises, it is possible to carry out efficient filtration without applying an elevated pressure as the filtration proceeds.
Owner:ASAHI KASEI MEDICAL CO LTD
Preparation method and device of leukocyte-depleted platelet-rich plasma
InactiveCN105107233BImprove production efficiencyEliminate the possibility of introducing contaminationCentrifugesLiquid separationWhole blood productWhite blood cell
The invention belongs to the technical field of blood products, and discloses a preparation method of leukocyte-depleted platelet-rich plasma. Secondary centrifugation forms a centrifuge that is divided into three layers from bottom to top to obtain the upper platelet and plasma components; (iii) centrifuge the obtained upper layer again to form the bottom platelet deposition and plasma supernatant; extract part of the plasma supernatant, The remaining plasma supernatant was used to resuspend platelet deposits to obtain leukocyte-depleted platelet-rich plasma. The method realizes the elimination of white blood cells and the enrichment of platelets at the same time, and is convenient to operate and takes a short time, and the technical indicators of the prepared plasma products are all better than the existing similar products. The two centrifugations in the method provided by the invention can be carried out in two centrifuge tubes respectively, or in the same centrifuge device, and the invention also provides a centrifuge device for implementing the preparation method.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Method for preparing a depleted plasma material consisting of one or more thrombogenic factors
InactiveUS20140044742A1Mammal material medical ingredientsPeptide preparation methodsFiltrationFractionation
The invention concerns a method for preparing a plasma product depleted of one or more thrombogenic factors, comprising the combination of at least two steps chosen from among an ethanol fractionation step, a filtration-adsorption step, a precipitation step with caprylic acid and a chromatography step on ion exchange resin.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Apparatus for cleaning reflective mask and method for cleaning reflective mask
ActiveCN106164771APhotomechanical apparatusSemiconductor/solid-state device manufacturingOrganic solventRuthenium
Owner:SHIBAURA MECHATRONICS CORP
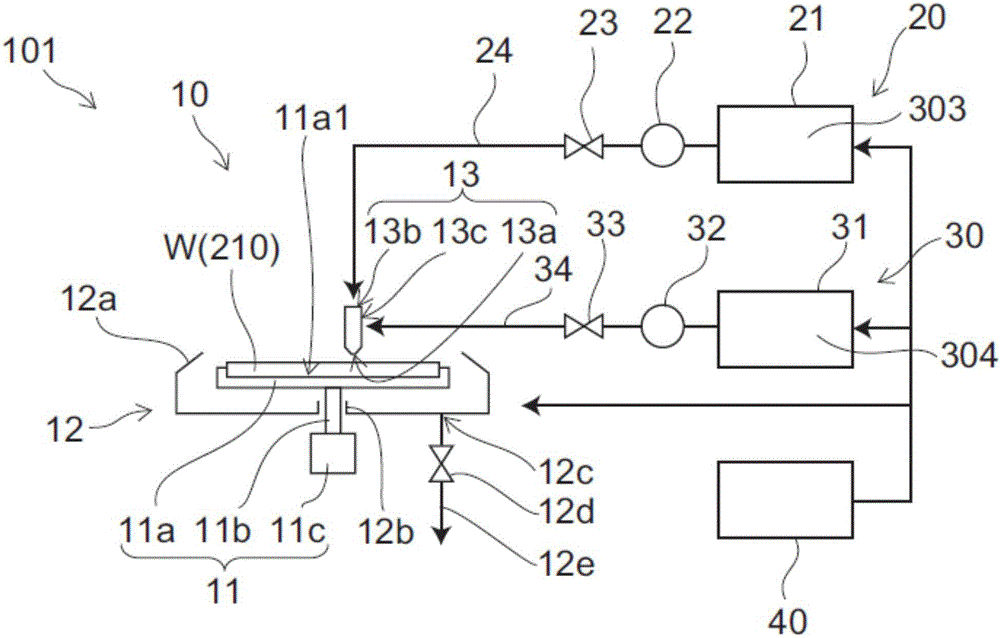
Optimal placement of a robust solvent/detergent process post viral ultrafiltration of an immune gamma globulin
The solvent-detergent (S / D) process is used to inactivate enveloped viruses in plasma products. While concentrations of 1.0% detergent and 0.3% tri-n-butyl phosphate solvent have been considered necessary for robust removal of viral activity, we show the effectiveness of solvent-detergent treatment after fractionation and nanofiltration of an immune gamma globulin preparation, which required significantly reduced concentrations of solvent and detergent. Reduced levels of solvent and detergent lead to greater efficiencies in their removal post-inactivation with the potential for greater yields and decreased processing costs.
Owner:ORTHO-CLINICAL DIAGNOSTICS
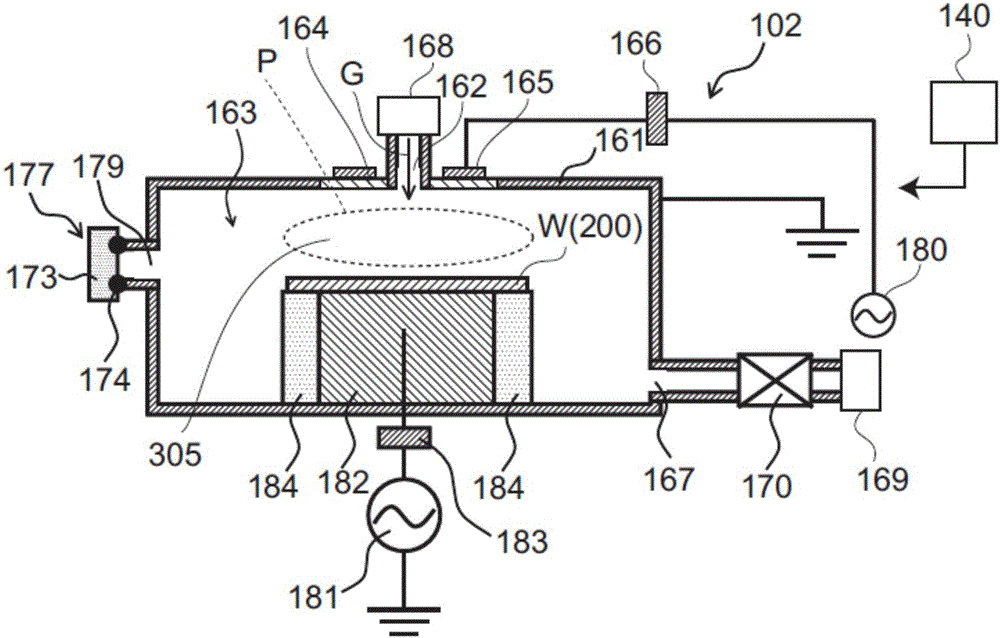
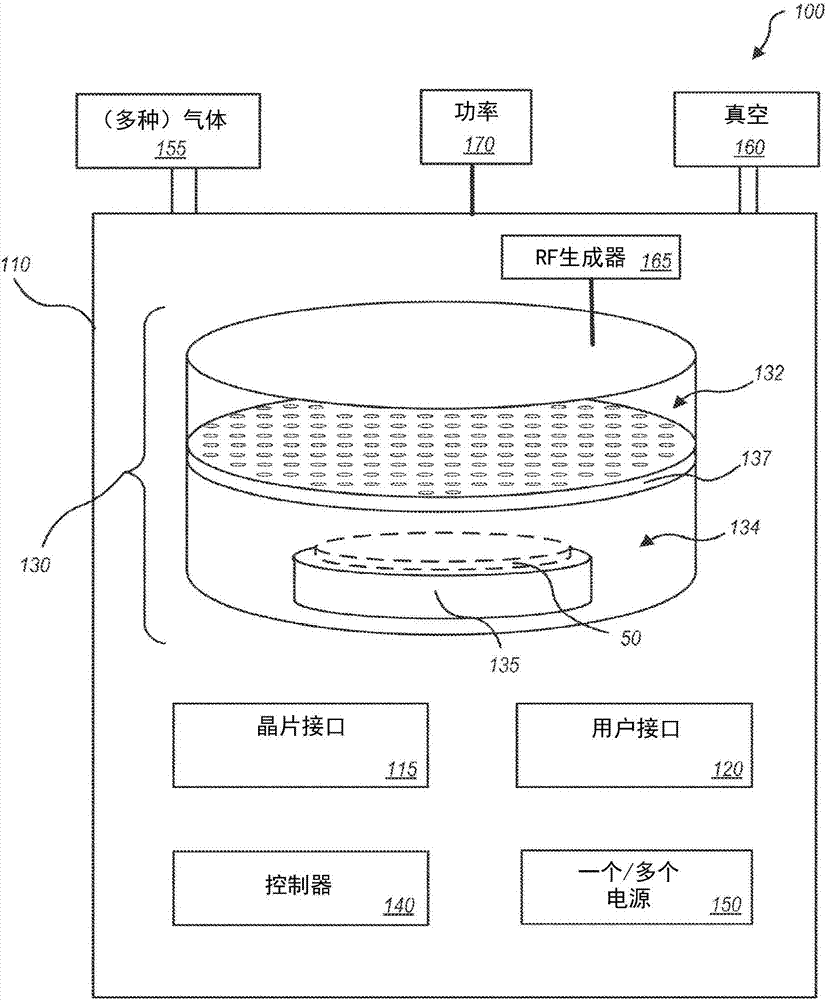
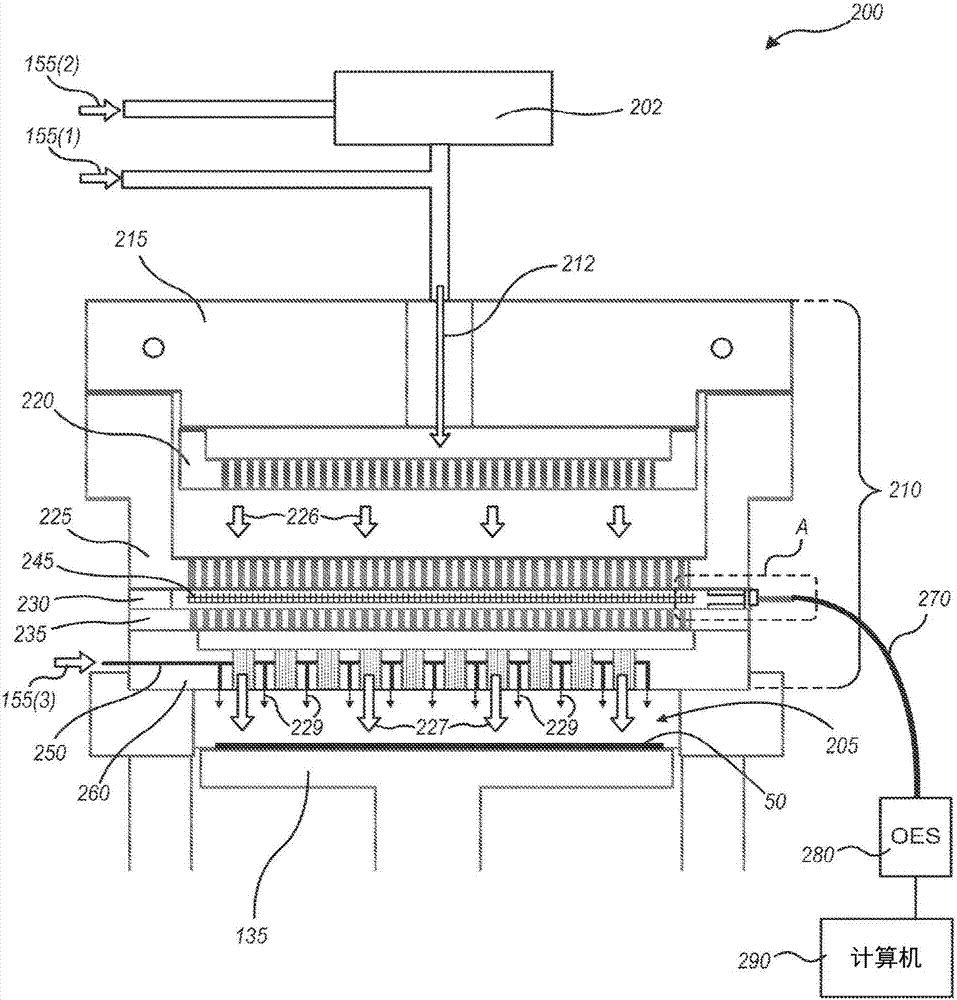
Systems and methods for internal surface conditioning assessment in plasma processing equipment
ActiveCN107078014AEmission spectroscopySemiconductor/solid-state device testing/measurementMaterial PerforationPlasma processing
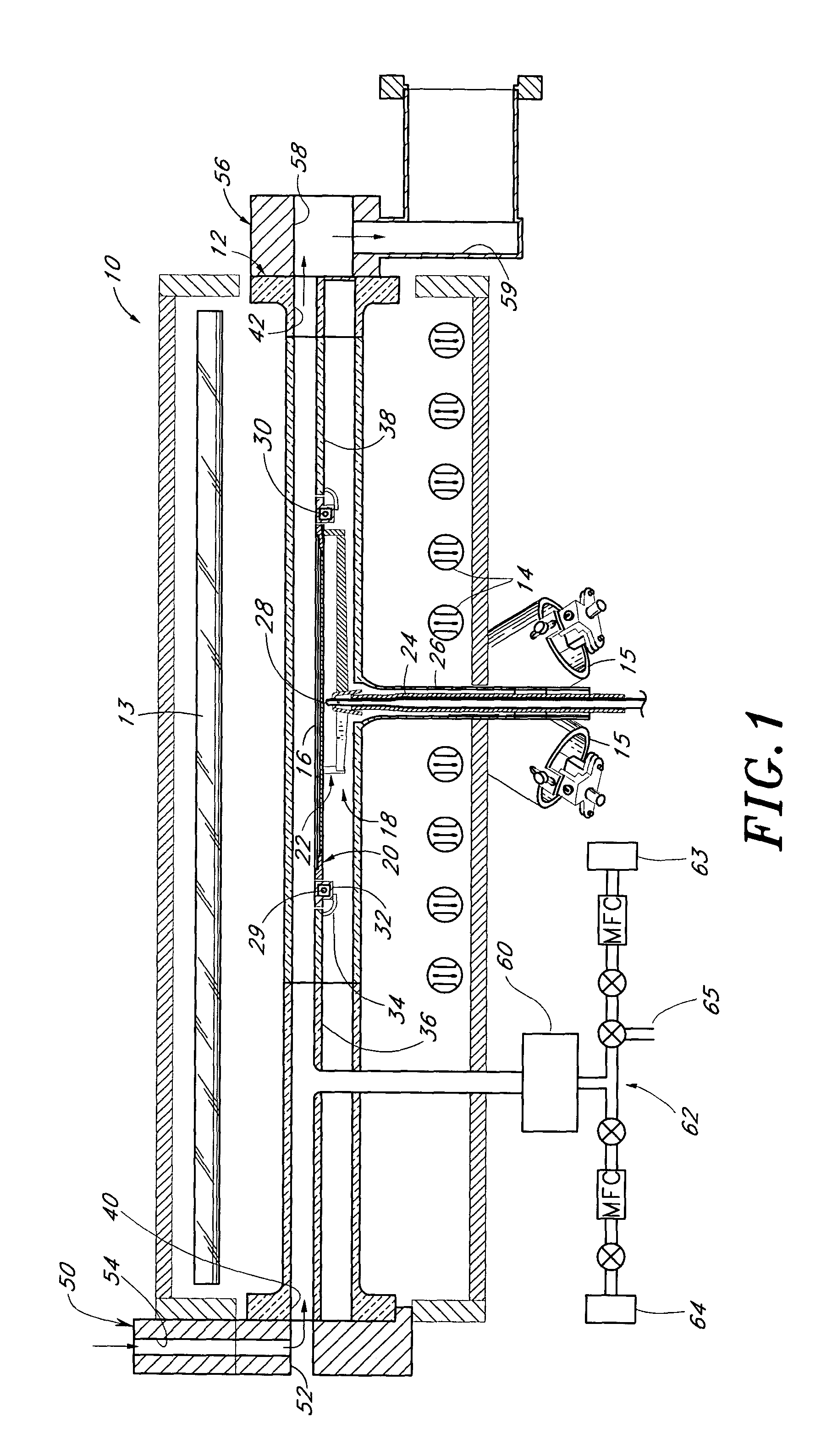
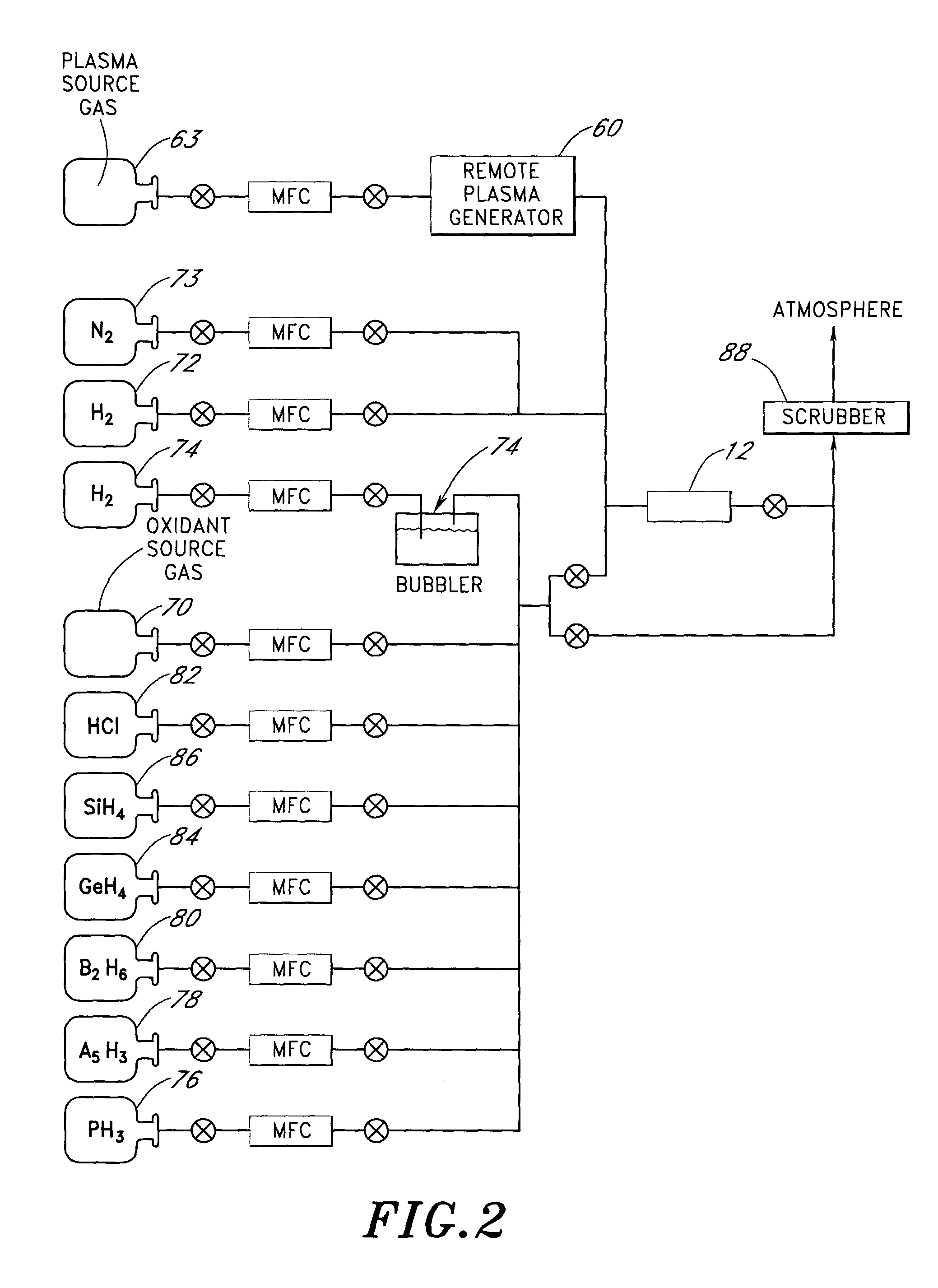
In an embodiment, a plasma source includes a first electrode, configured for transfer of one or more plasma source gases through first perforations therein; an insulator, disposed in contact with the first electrode about a periphery of the first electrode; and a second electrode, disposed with a periphery of the second electrode against the insulator such that the first and second electrodes and the insulator define a plasma generation cavity. The second electrode is configured for movement of plasma products from the plasma generation cavity therethrough toward a process chamber. A power supply provides electrical power across the first and second electrodes to ignite a plasma with the one or more plasma source gases in the plasma generation cavity to produce the plasma products. One of the first electrode, the second electrode and the insulator includes a port that provides an optical signal from the plasma.
Owner:APPLIED MATERIALS INC
Purification method of human fibrinogen
The invention discloses a purification method of human fibrinogen and belongs to the field of plasma product preparation processes. The method comprises the following step: carrying out ion exchange chromatography on fibrinogen subjected to virus inactivation, thereby obtaining purified fibrinogen, wherein a buffer solution adopted by the ion exchange chromatography comprises an equilibrium buffer solution, a washing buffer solution and an eluting buffer solution. Experimental results show that by means of the purification method disclosed by the invention, the content and purity of the human fibrinogen in an obtained product can be obviously improved, and virus inactivator residues in the product can be obviously reduced. The method disclosed by the invention is simple and convenient to operate and low in cost and has a good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
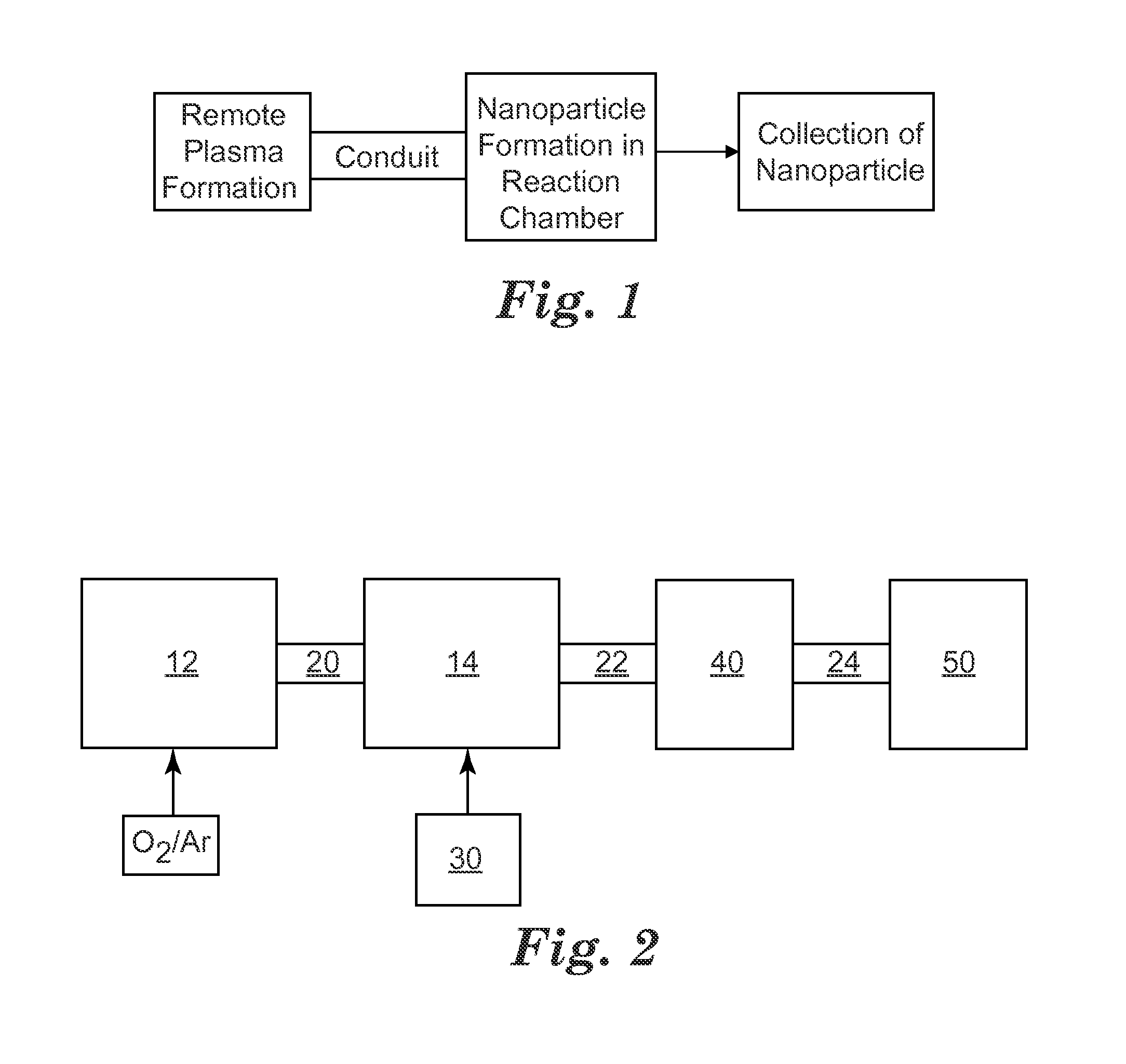
Remote plasma synthesis of metal oxide nanoparticles
A method of synthesizing nanoparticles, comprising providing a precursor comprising a titanium alkoxide compound; forming a plasma from oxygen gas at a first location, wherein the plasma comprises plasma products that contain oxygen atoms; causing the plasma products to flow to a second location remote from the first location; contacting the precursor with the plasma products at the second location so as to oxidize the precursor and form nanoparticles; and collecting the nanoparticles with a collector.
Owner:3M INNOVATIVE PROPERTIES CO
Low pressure generating plasma reactor closed loop process and system
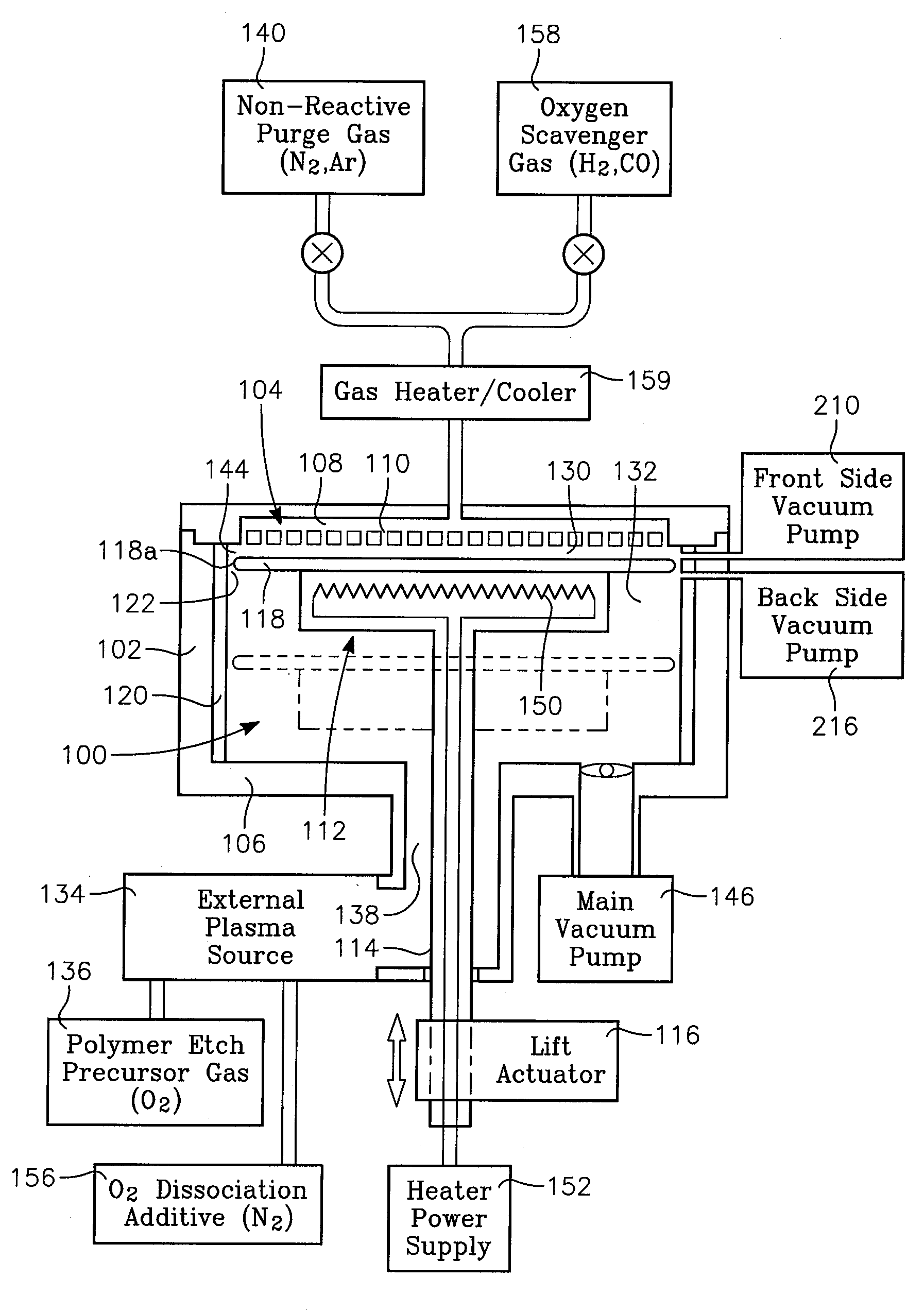
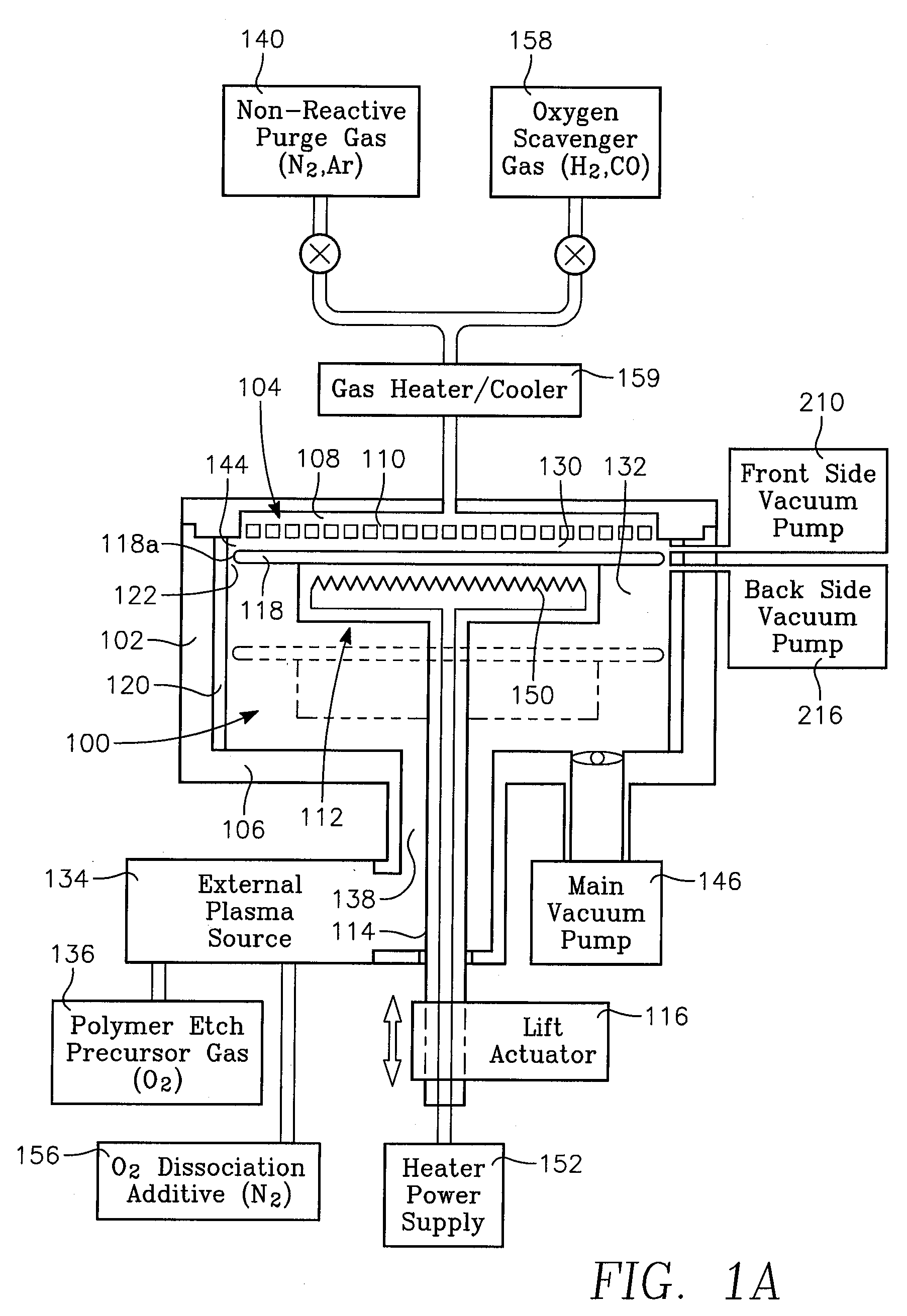
PendingUS20210402362A1Reduce pressureAvoid accumulationProcess control/regulationExcrement fertilisersPhysical chemistryReactive plasma
The present invention provides a low pressure generating plasma reactor closed loop process, comprising: feeding a fresh feed gas flow and a fresh feed absorption liquid flow to a plasma reactor closed loop comprising a condenser, a liquid loop, a recycle gas loop, and a plasma generator; converting feed gas to reactive plasma products in the plasma generator; quenching and absorbing the reactive plasma products into an absorption liquid circulating in the liquid loop where the reactive plasma products react to form liquid reaction products, thereby generating low pressure in the closed loop; monitoring the composition and low pressure of the recycle gas loop and, if the pressure increases, adjusting the composition of the fresh feed gas flow and / or fresh feed absorption liquid flow to bring the composition of the feed gas towards stoichiometric ratio with the absorbed reactive plasma products; extracting circulating absorption liquid, containing the liquid reaction products, from the plasma reactor closed loop as a product flow. The present invention also provides a low pressure generating plasma reactor closed loop system, comprising a plasma generator, a condenser, a recycle gas loop, a liquid loop, and a pump.
Owner:N2 APPLIED
Virus Inactivation Monitor
ActiveCN108444854BSituations to Avoid Weighing WorkSmall footprintLavatory sanitoryMaterial weighingThermodynamicsPlasma bag
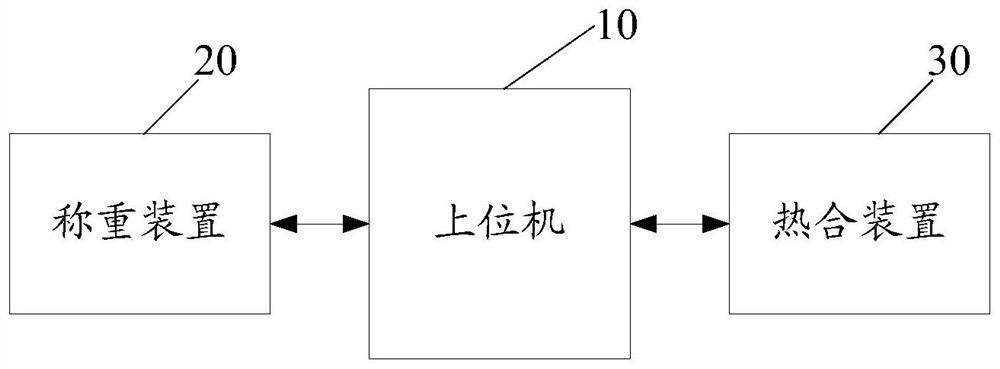
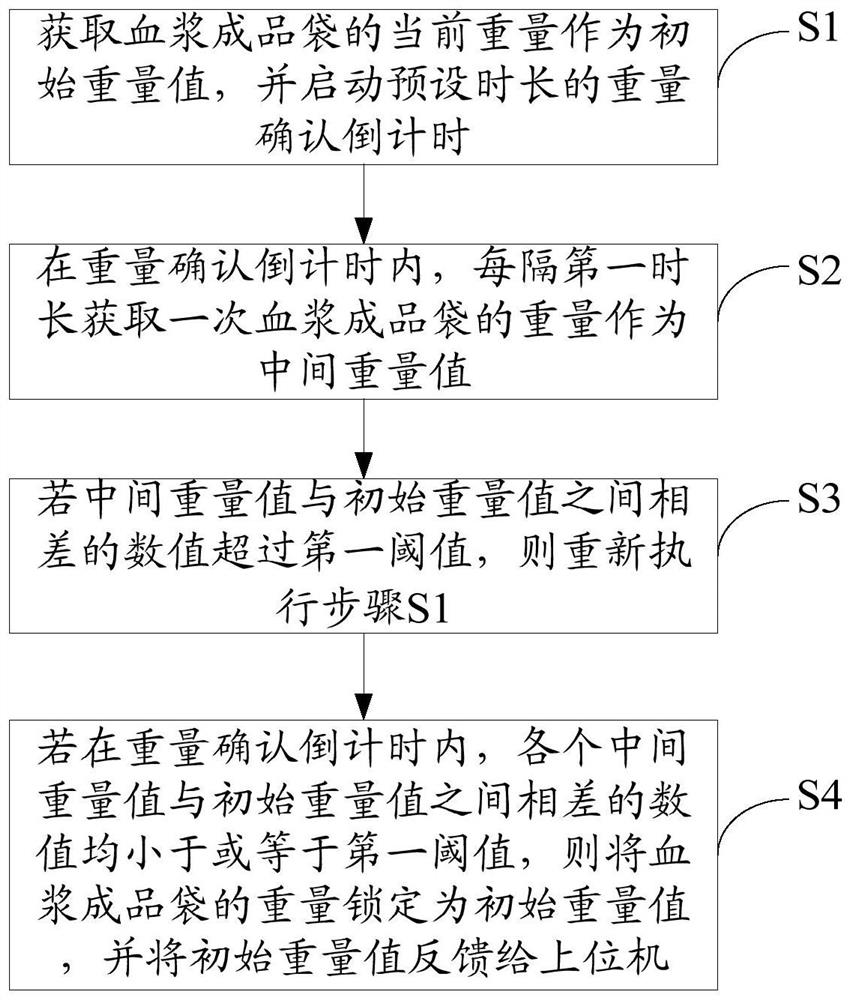
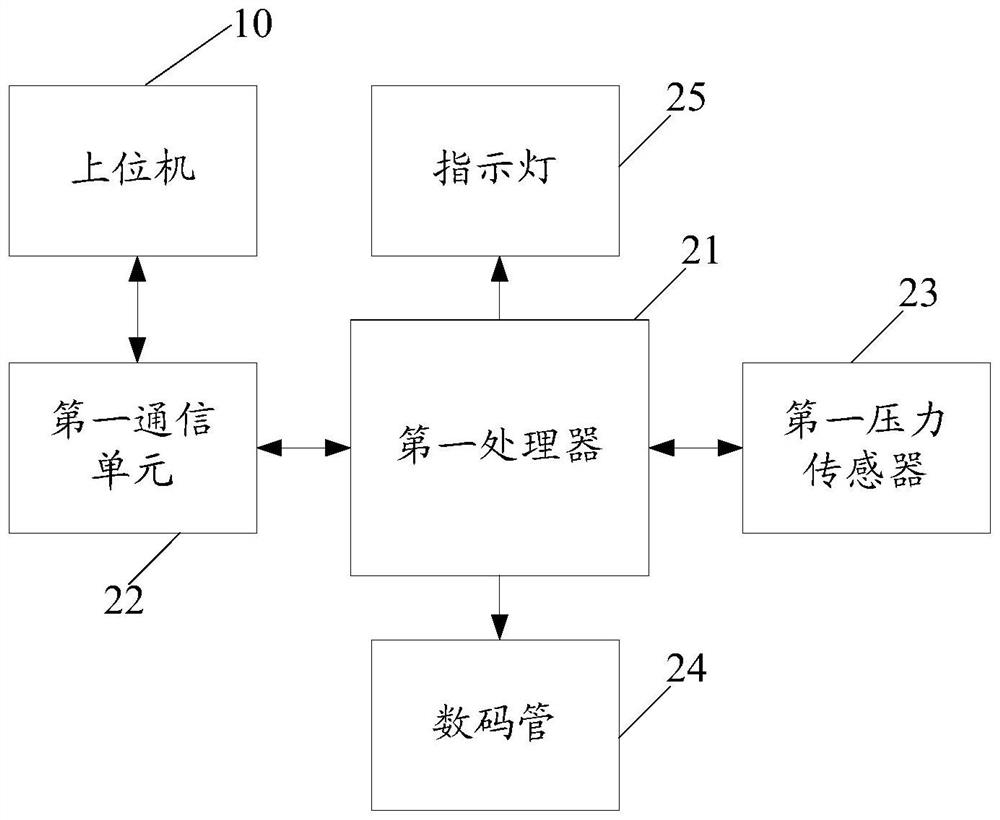
The invention discloses a virus inactivation monitor, comprising a body, a host computer, a filter support device, a weighing device and a heat sealing device arranged on the body, and the filter support device is used for hanging in the plasma virus inactivation filtration process and supporting the blood bag; the upper position electromechanically connects the weighing device and the heat sealing device, and the weighing device is used to weigh the finished plasma bag according to the weighing instruction issued by the upper computer, and lock and weigh it after the weight of the finished plasma bag is stable. After the weight of the finished plasma bag is locked by the weighing device, the upper computer controls the heat sealing device to heat seal the inlet and outlet pipes of the finished plasma bag; the upper computer controls the weighing after the heat sealing device completes the heat sealing of the inlet and outlet pipes of the finished plasma bag. The unit releases the weight lock to weigh the next plasma product bag. The virus inactivation monitor of the present invention ensures the stability of weighing and heat-sealing operations, reduces the space occupied by the equipment, and improves the overall efficiency during the production process of the plasma product bag.
Owner:深圳市迈思特生物医学工程有限公司
Blood coagulation factor type plasma quick-freezing cold storage
PendingCN114087828AEvenly distributedPrevent leakageLighting and heating apparatusStationary refrigeration devicesCold airTemperature control
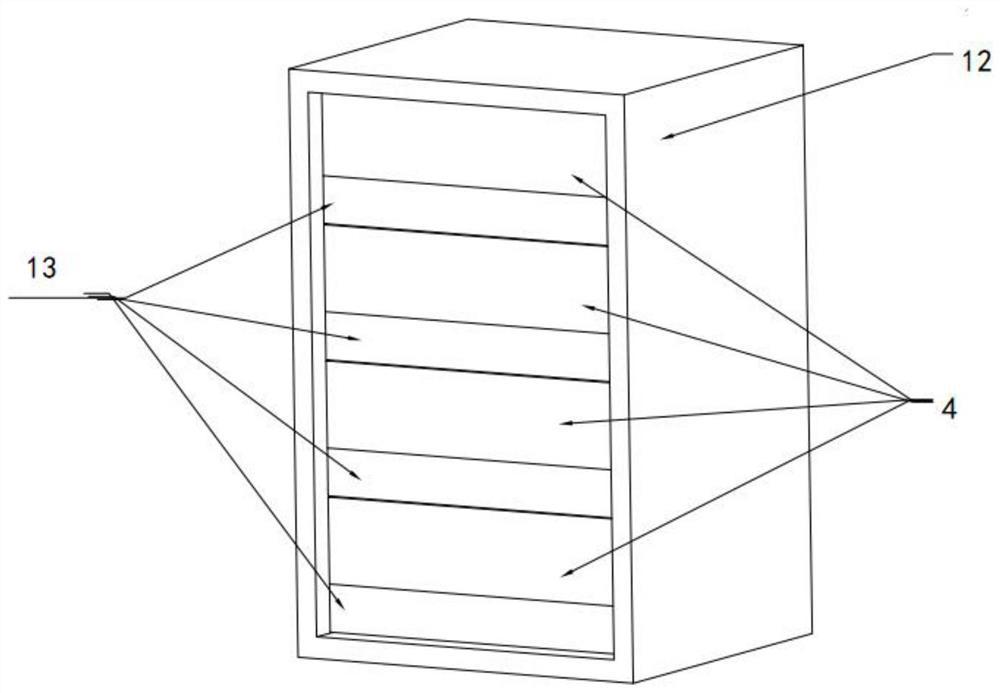
The invention provides a blood coagulation factor type plasma quick-freezing cold storage which comprises a cold storage body and at least one refrigerating unit, each refrigerating unit comprises a cold storage door, quick-freezing flat plates, a fan system, an evaporator and shielding parts, the cold storage door is arranged on the front side of the refrigerating unit, one side of the cold storage door is the shielding part, and the other side of the cold storage door is the external environment. Each shielding part comprises an inner door and a connecting plate. The quick-freezing flat plates are fixed to the supporting frame and are arranged in multiple layers, and a gap is formed between every two adjacent quick-freezing flat plates to form a containing space. The shielding parts are used for sealing the containing spaces corresponding to the quick-freezing flat plates, and the interior of the refrigerating unit is divided into a plurality of independent sealed containing spaces. When the cold storage door is opened to take plasma products, only the corresponding shielding part on a certain layer needs to be opened, leakage of most cold air is avoided, more accurate temperature control is facilitated, temperature fluctuation is reduced, and quick freezing and activity of stored blood coagulation factor plasma are facilitated.
Owner:HUALAN BIOLOGICAL ENG INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com