Trifluoromethyl-containing all-carbon quaternary carbon center indole acetonitrile compound as well as preparation method and application thereof
A technology of trifluoromethyl all-carbon quaternary carbon and fluoromethyl indole alcohol, which is applied in the field of indole acetonitrile compounds containing trifluoromethyl all-carbon quaternary carbon centers and its preparation, and can solve the problems of synthesis without literature reports, etc. Achieve the effects of wide reaction substrate range, strong inhibitory effect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The reaction equation is as follows:
[0040]
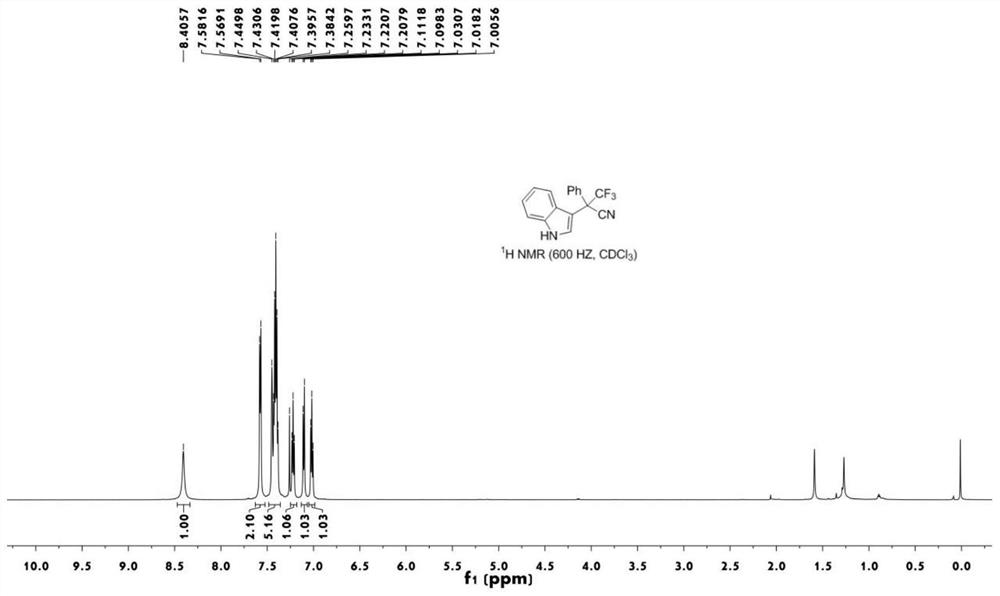
[0041] Take a 10mL round bottom flask, add trifluoromethyl indole alcohol 2a (0.3mmol), tris(pentafluorophenyl)borane (0.03mmol), add chlorobenzene (3mL), trimethylsilyl cyanide (0.9mmol) in turn ), stirred and reacted at 120°C for 8 hours (thin plate chromatography tracked the reaction until the reaction was complete), after the reaction was completed, cooled to room temperature, the reaction mixture was concentrated by rotary evaporation to remove the solvent, and the crude product was separated by silica gel column chromatography (eluent was petroleum ether : ethyl acetate=25 / 1~8 / 1, V / V), the target product 1a (89.2 mg, white solid, yield 99%) was obtained.
[0042] figure 1 The proton nuclear magnetic resonance spectrum figure of the compound 1a prepared for embodiment;
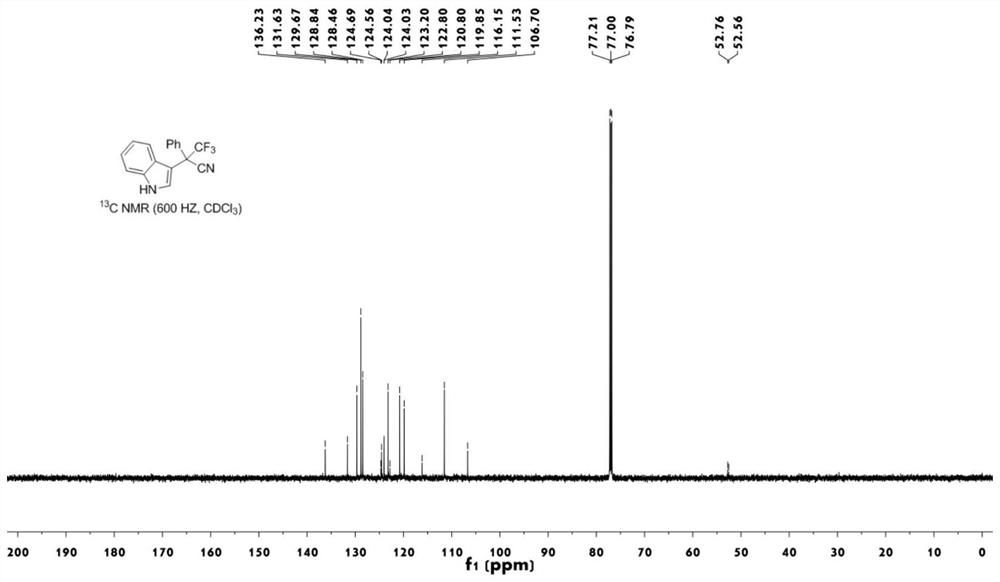
[0043] figure 2 The carbon nuclear magnetic resonance spectrum of the compound 1a prepared for the embodiment;
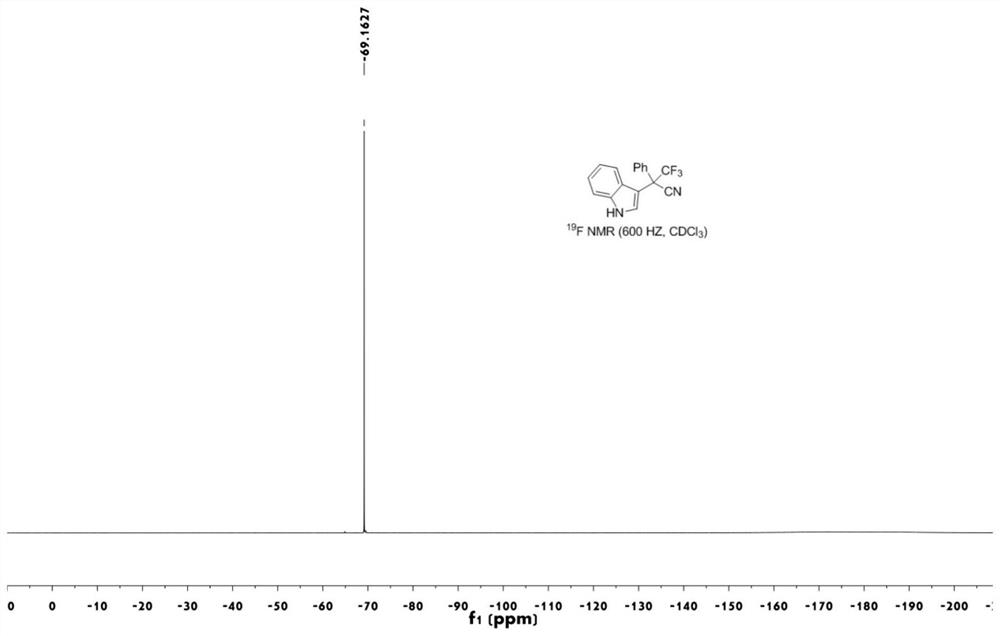
[0044] image 3 The fluorine ...
Embodiment 2
[0048] The reaction equation is as follows:
[0049]
[0050] Take a 10mL round bottom flask, add trifluoromethylindole alcohol 2b (0.3mmol), tris(pentafluorophenyl)borane (0.03mmol), add chlorobenzene (3mL), trimethylsilyl cyanide (0.9mmol) in turn ), stirred and reacted at 120°C for 15 hours (thin plate chromatography tracked the reaction until the reaction was complete), after the reaction was finished, cooled to room temperature, the reaction mixture was concentrated by rotary evaporation to remove the solvent, and the crude product was separated by silica gel column chromatography (eluent was petroleum ether : ethyl acetate=25 / 1~8 / 1, V / V), the target product 1b (91.9 mg, white solid, yield 99%) was obtained.
[0051] 1 H NMR (600MHz, CDCl 3 )δ8.69(s,1H),7.50(d,J=7.6Hz,2H),7.47–7.43(m,1H),7.43–7.33(m,3H),7.19(d,J=8.2Hz,1H ),7.12(td,J=8.0,4.8Hz,1H),6.66(dd,J=11.0,7.8Hz,1H); 13 C NMR (101MHz, CDCl 3 )δ155.4(d, J=248.6Hz), 138.8(d, J=10.4Hz), 132.5(d, J=1.2Hz), 129.5,...
Embodiment 3
[0053] The reaction equation is as follows:
[0054]
[0055] Take a 10mL round bottom flask, add trifluoromethyl indole alcohol 2c (0.3mmol), tris(pentafluorophenyl)borane (0.03mmol), add chlorobenzene (3mL), trimethylsilyl cyanide (0.9mmol) in turn ), stirred and reacted at 120°C for 12 hours (thin plate chromatography tracked the reaction until the reaction was complete), after the reaction was finished, cooled to room temperature, the reaction mixture was concentrated by rotary evaporation to remove the solvent, and the crude product was separated by silica gel column chromatography (eluent was petroleum ether : ethyl acetate=25 / 1~8 / 1, V / V), the target product 1c (90.7 mg, white solid, yield 99%) was obtained.
[0056] 1 H NMR (400MHz, CDCl 3 )δ8.39(s,1H),7.62(d,J=7.3Hz,2H),7.49–7.33(m,4H),7.29(d,J=8.3Hz,1H),7.07(d,J=8.3 Hz,1H),6.95(s,1H),2.32(s,3H); 13 C NMR (101MHz, CDCl 3 )δ134.5, 131.6, 130.1, 129.6, 128.8, 128.4, 124.8, 124.7, 124.1(q, J=2.6Hz), 123.8(q, J=286...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com