Method for preparing remimazolam
A technology of remimazolam and an intermediate, which is applied in the field of preparation of labor pain anesthetic drug remazolam, can solve problems such as waste, and achieve the effects of improving quality, optimizing preparation process and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] first step:
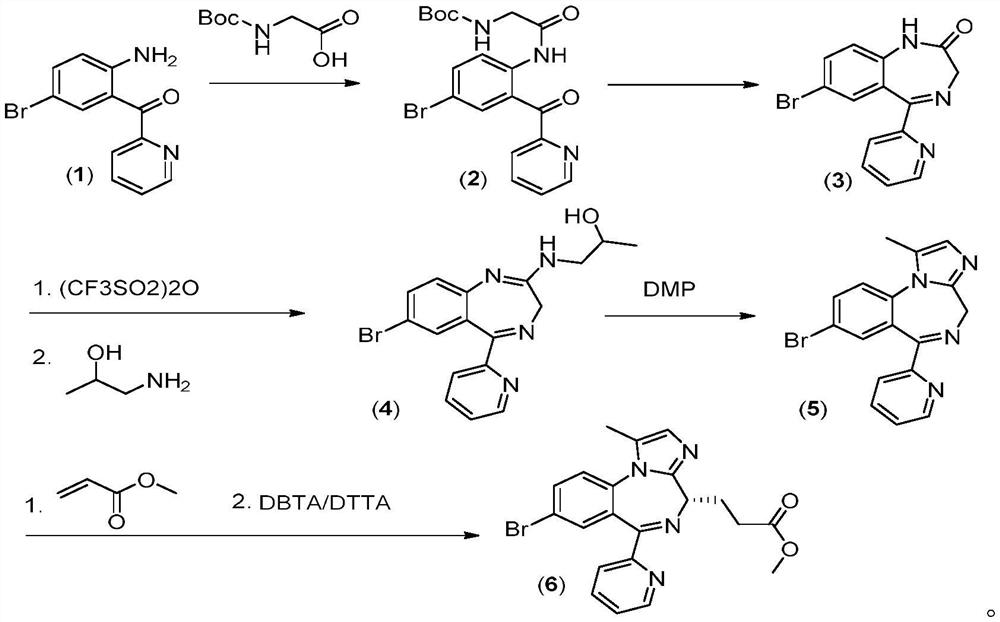
[0046] 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.9g, 4.7mmol) was added to BOC-glycine (0.63g, 3.61mmol) and 2-(2-amino- 5-Bromo-benzoyl)pyridine (compound (1)) (1.0g, 3.61mmol) / tetrahydrofuran (16mL) solution, stirred at room temperature for 6 hours, concentrated under reduced pressure, added ethyl acetate and water to extract the reaction. The organic layer was washed with brine, dried and spin-dried to obtain compound 2 (1.46 g, yield 93.0%).
[0047] Step two:
[0048]Pass hydrogen chloride gas into the compound 2 (1.46g, 3.36mmol) / methanol (15mL) solution, stop the ventilation after 20 minutes, stir the solution at room temperature overnight, wash with aqueous sodium bicarbonate, and spin the organic layer to dryness with aqueous sodium bicarbonate (1N) Adjust pH=8-9, extract with dichloromethane 3 times, spin dry and recrystallize with methanol / water to obtain compound 3 (0.90 g, yield: 84.9%).
[0049] The third step: 7-bromo...
Embodiment 2
[0057] first step:
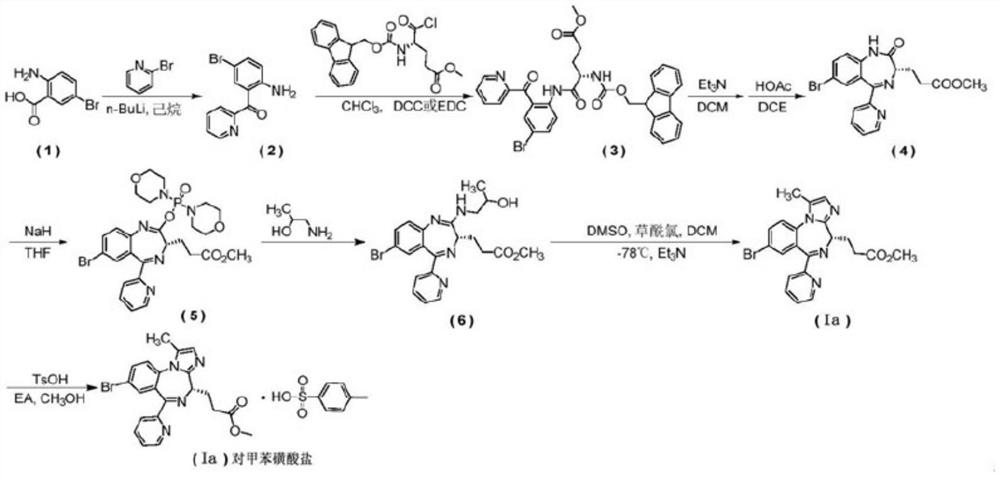
[0058] 3,5-Dinitrophenylboronic acid (0.153kg, 0.72mol) was added to BOC-glycine (0.69kg, 3.97mol) and 2-(2-amino-5-bromo-benzoyl)pyridine (compound (1) ) (1kg, 3.61mol) / dioxane (13L) / toluene (4L) solution, reflux and water separation for 6 hours, concentrate under reduced pressure and then add ethyl acetate and water to extract the reaction. The organic layer was washed with brine, dried and spin-dried to obtain compound 2 (1.48 kg, yield 94.3%).
[0059] Step two:
[0060] Add trifluoroacetic acid (0.58kg, 5.11mol) into a solution of compound 2 (1.48kg, 3.41mol) in dioxane (7L), stir the solution overnight at room temperature, wash with aqueous potassium carbonate solution, spin the organic layer to dryness, and use Potassium carbonate aqueous solution (1N) was used to adjust the pH to 8-9, extracted three times with dichloromethane, spin-dried and then recrystallized with methanol / water to obtain compound 3 (0.92kg, yield 85.1%).
[0061] The third s...
Embodiment 3
[0069] first step:
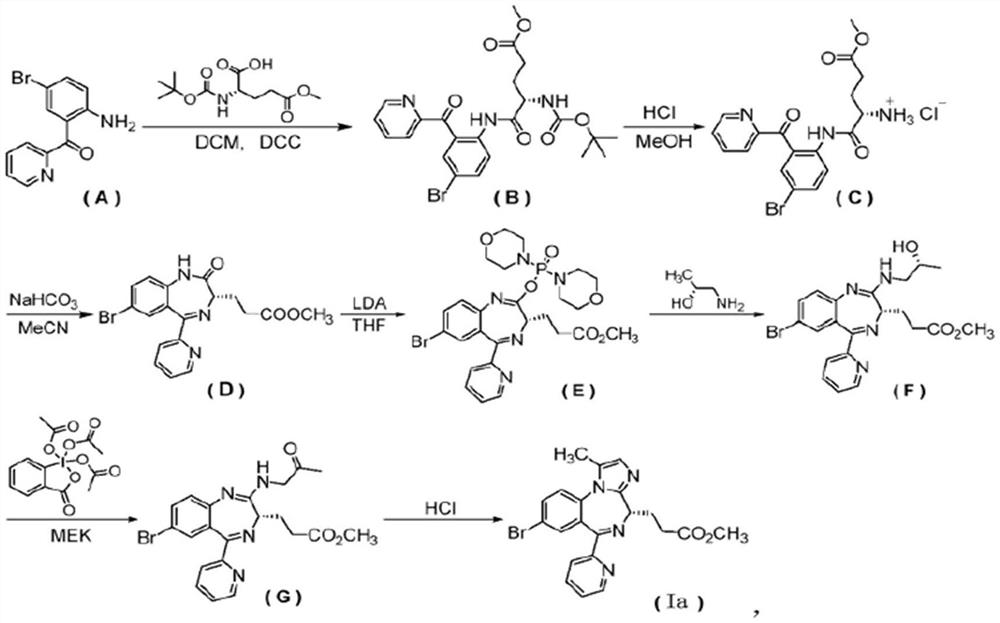
[0070] Tris(2,2,2-trifluoroethyl) borate (1.67kg, 5.41mol) was added to BOC-glycine (6.64kg, 37.89mol) and 2-(2-amino-5-bromo-benzoyl) Pyridine (compound (1)) (10kg, 36.09mol) / dioxane (140L) / toluene (30L) solution, refluxed for 6 hours, concentrated under reduced pressure, added ethyl acetate and water to extract the reaction. The organic layer was washed with brine, dried and spin-dried to obtain compound 2 (14.42 kg, yield 92.0%).
[0071] Step two:
[0072] Concentrated hydrochloric acid (4.15L, 49.8mol) was added in the ethyl acetate (75L) solution of compound 2 (14.42kg, 33.20mol), and the solution was stirred overnight at room temperature, washed with an aqueous solution of DBU, and the organic layer was spin-dried with an aqueous solution of DBU ( 1N) adjust the pH to 8-9, extract with dichloromethane three times, spin dry and recrystallize with methanol / water to obtain compound 3 (9.01kg, yield: 85.8%).
[0073] The third step: 7-bromo-2-(2-hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com