Nir/pet bimodal probe precursor targeting cyp1b1 enzyme and its preparation and use
A dual-mode, probe technology, applied in the direction of in vivo radioactive preparations, in vivo test preparations, radioactive carriers, etc., can solve the problem of low diagnostic specificity, difficult diagnosis, and near-infrared single-mode molecular probe penetration Problems such as limited depth, to achieve the effect of deep non-destructive testing, large detection depth, good application prospects and clinical transformation value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
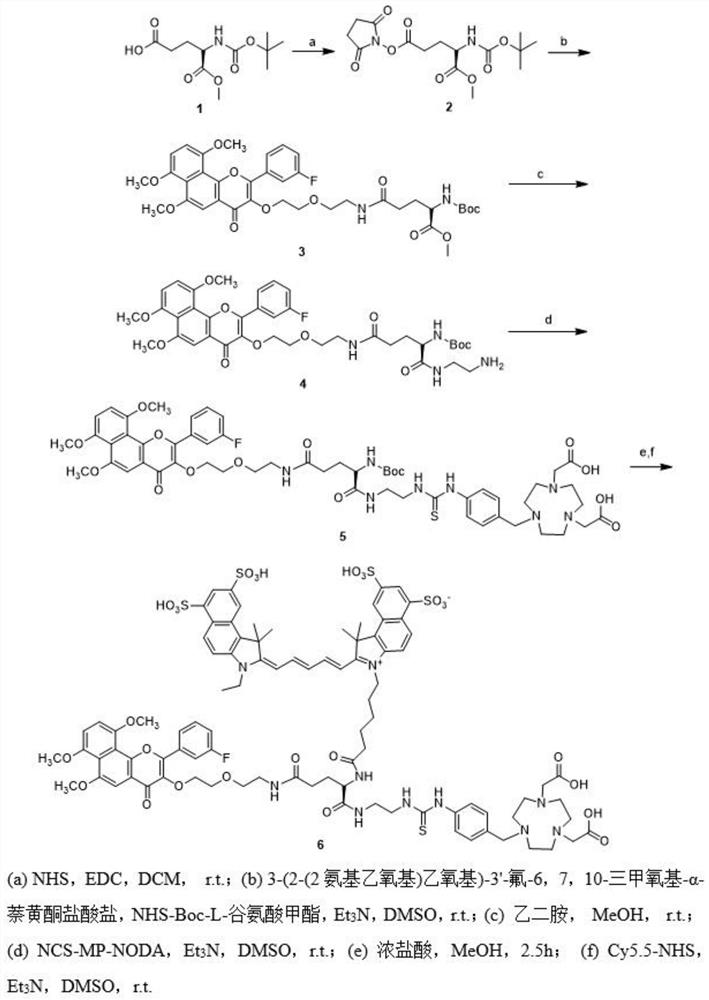
[0049] This embodiment relates to a method for preparing a NIR / PET bimodal probe with structural formula 6. The synthetic route is as follows figure 1 shown, including the following steps:
[0050] Step 1: Boc-L-glutamic acid methyl ester (1 g, 3.8 mmol) and N-hydroxysuccinimide (NHS) (440 mg, 3.8 mmol) were dissolved in 7 mL of tetrahydrofuran (THF), and then added and dissolved in 5 mL of 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) (594 mg, 3.8 mmol) in dichloromethane (DCM). The reaction was mixed and stirred overnight and monitored by thin layer chromatography (TLC). After completion of the reaction, the solvent was evaporated under reduced pressure and the residue was dissolved in 20 mL of DCM. After that, the solution was washed with water, saturated NaHCO 3 and brine wash. After drying the organic phase with magnesium sulfate, it was concentrated under reduced pressure to obtain compound 2 as a white solid product, yield: 95%. 1 H NMR (400MHz, CDCl 3 ): 5...
Embodiment 2
[0056] The inhibitory activity of compound 5 obtained in Example 1 on CYP1A1, CYP1A2, and CYP1B1 enzymes was determined.
[0057] In this experiment, 7-ethoxy-3H-phenoxazine 3-one deethoxylation (EROD) assay was used to determine its inhibitory activity and selectivity to CYP1A1, CYP1A2, and CYP1B1 enzymes (Yamaori et al, Biochem. Pharmacol. 2010 , 79:1691-1698.). The reaction system (200 μL) contained CYP1A1 (10 fmol), CYP1A2 (60 fmol) or CYP1B1 (20 fmol), different concentrations of the test compound, NADPH regeneration system (1.3 mM NADPNa2, 3.3 mM glucose-6-phosphate, 0.5 U / ml glucose- 6-phosphate-dehydrogenase), 3.3 mM magnesium chloride solution and 150 nM 7-ethoxy-3H-phenoxazin-3-one. Four replicate wells were set up in each experimental group or control group as a parallel experiment. The reaction buffer was 50 mM Tris-HCl (pH 7.4) buffer containing 1% BSA solution. After the reaction system was preheated at 37°C for 5 min, the NADPH regeneration system was added t...
Embodiment 3
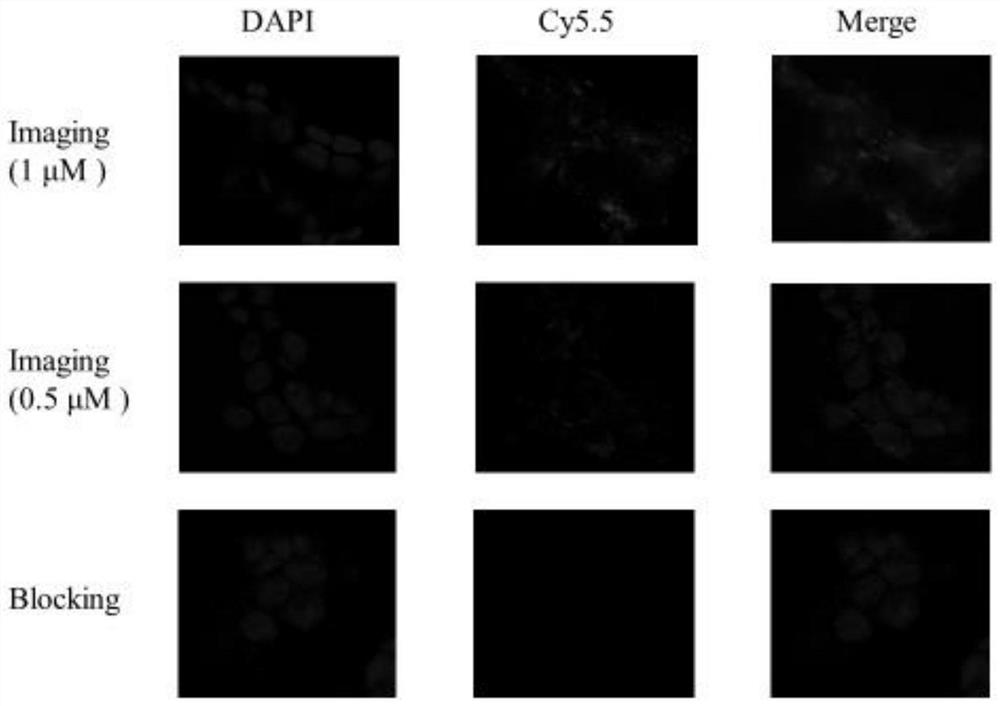
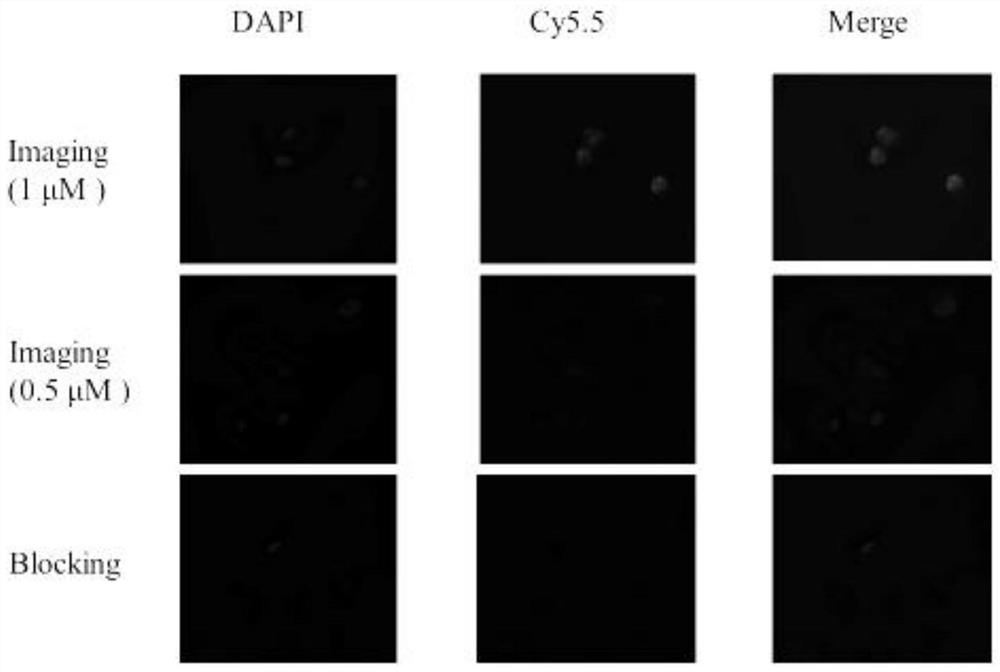
[0062] In this example, the confocal microscopy imaging study of the NIR / PET dual-modality molecular probe precursor (compound 6) on the colon cancer cell HCT-15 with high expression of CYP1B1 enzyme was carried out.
[0063] HCT-15 colon cancer cells with high expression of CYP1B1 enzyme were seeded in 8-well Nunc at appropriate density TM Lab-Tek TM Chamber slide system, in a cell incubator at 37 °C, 5% CO 2 After culturing in medium for 24h, aspirate the excess medium, add 2*1μM and 2*0.5μM NIR / PET dual-modality molecular probe precursors respectively, and set up a blocking control group (the blocking group adds 2*1μM probe and 20 μM solution of α-naphthoflavone derivatives), 2 duplicate wells in each group. 37°C, 5% CO 2 After 1 hour incubation in medium, the probe-containing medium was aspirated and washed three times with PBS buffer. Afterwards, the chamber on the slide was removed according to the instructions, and the sealing oil containing the nuclear dye DAPI was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com