Method for preparing hydroxyl group-containing olefin copolymer and product and application thereof
A technology of olefin copolymer and hydroxyl group, which is applied in the field of polymer polymer preparation, and achieves the effects of improving spheroidizing effect, good appearance and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
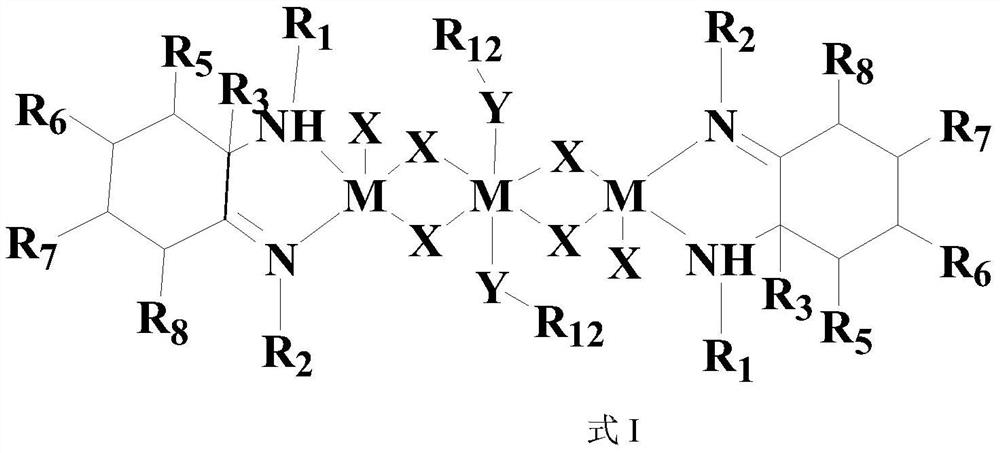
[0134] 1) Preparation of Ligand L1:
[0135]1.5ml 2,6-dimethylaniline (12mmol) was reacted with 57ml 1M trimethylaluminum in toluene, after reflux for 3h, camphorquinone (1.05g, 5mmol) was added, and the reaction was reflux for 8 hours. The reaction was terminated with sodium / ice water, extracted with ethyl acetate, the organic phases were combined, and dried over anhydrous magnesium sulfate. The product was separated by petroleum ether / ethyl acetate column chromatography to obtain a colorless crystal ligand L1 with a yield of 70.2%. 1 HNMRδ(ppm)7.00-6.89(m,6H,Ar-H),3.57(s,1H,NH),2.18(s,6H,CAr-CH 3 ),2.05(s,6H,CH 3 ),1.74(m,4H,CH 2 ),1.44(s,3H,CH 3 ),1.35(m,1H),1.21(s,3H,CH 3 ),1.01(s,3H,CH 3 ),0.87(s,3H,CH 3 ).
[0136] 2) Preparation of complex Ni1: 10ml (DME) NiBr 2 (277mg, 0.9mmol) in ethanol (10mL) was added dropwise to 10ml of ligand L1 (233mg, 0.6mmol) in dichloromethane (10mL), stirred at room temperature for 6 hours, a precipitate precipitated, filtered, washe...
Embodiment 2
[0139] Dry the 1L stainless steel polymerization kettle equipped with mechanical stirring at 130 ° C for 6 hours, vacuumize while it is hot and use N 2 Air replacement 3 times. Inject 500 mL of hexane into the polymerization system, and simultaneously add 7.6 mg (5 μmol) of the complex Ni1, 50 mL of dichloromethane, 30 mmol (5.1 mL) of 2-methyl-2-hydroxy-7-octene, 30 mL of AlEt 3 (1.0mol / L hexane solution), 6.5mL MAO (1.53mol / L toluene solution), at 30°C, keep 10atm ethylene pressure, and stir for 30min. Finally, it was neutralized with an ethanol solution acidified with 10 wt% hydrochloric acid to obtain a polymer. The polymerization activity and the performance parameters of the polymer are shown in Table 1.
Embodiment 3
[0141] Dry the 1L stainless steel polymerization kettle equipped with mechanical stirring at 130 ° C for 6 hours, vacuumize while it is hot and use N 2 Air replacement 3 times. Inject 500 mL of hexane into the polymerization system, and simultaneously add 7.6 mg (5 μmol) of the complex Ni1, 100 mL of dichloromethane, 30 mmol (5.1 mL) of 2-methyl-2-hydroxy-7-octene, 30 mL of AlEt 3 (1.0mol / L hexane solution), 6.5mL MAO (1.53mol / L toluene solution), at 60°C, keep 10atm ethylene pressure, and stir for 30min. Finally, it was neutralized with an ethanol solution acidified with 10 wt% hydrochloric acid to obtain a polymer. The polymerization activity and the performance parameters of the polymer are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com