Protein tyrosine phosphatase selective inhibitor, application, preparation method and pharmaceutical composition
A tyrosine phosphatase, selective technology, applied in the field of protein tyrosine phosphatase selective inhibitors, can solve the problems of affecting the metabolic process, high similarity of enzyme active centers, adverse drug reactions, etc., to improve enzyme inhibition Activity, the effect of reducing the effective use concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, the preparation method of GQDs
[0036] Graphene (0.15 g) was added to a mixture of concentrated sulfuric acid (30 mL) and concentrated nitric acid (10 mL). After the mixed solution was sonicated for 2 h, the mixture was put into a polytetrafluoroethylene-lined reactor and reacted at 120° C. for 5 h. After the reaction, the reaction solution was cooled to room temperature and diluted to 300 mL with deionized water. The pH was adjusted to about 8 with solid anhydrous sodium carbonate. The reaction solution was placed at 4 ° C for 24 h. After the supernatant was filtered through a 0.22 μm water-based filter membrane, it was dialyzed with a 1000 Da dialysis bag at room temperature for 2 days (changing water 6 times a day) to remove inorganic salt ions to obtain a light brown clear solution, which was then freeze-dried to obtain a loose brown-yellow solid .
Embodiment 2
[0037] Embodiment 2, the preparation method of PTP1B selective ligand
[0038]
[0039] 500 mg of 2,6-dihydroxybenzoic acid was dissolved in 5 mL of methanol, and 5 mL of methanol solution containing 323.6 μL of thionyl chloride was slowly added dropwise under ice cooling. After the dropwise addition was completed, it was heated to 60° C. for 2 h. The reaction was detected by TLC. After the reaction was completed, the reaction liquid was removed by rotary evaporation to obtain the crude product, which was recrystallized in methanol to obtain the pure intermediate 1.
[0040]
[0041] 400mg of intermediate 1, 342.8mg of N-Boc-4-aminobutanol, and 652.7mg of triphenylphosphine were suspended in 20mL of tetrahydrofuran, and under nitrogen protection, diethyl azodicarboxylate (368 μL diluted in 2mL tetrahydrofuran), reacted for 1h, and after the reaction was completed, diluted the reaction solution with ethyl acetate, extracted the reaction solution with water three times, col...
Embodiment 3
[0046] Example 3. Preparation method of GQDs-based vanadium coordination complex with PTP1B selective inhibitory activity
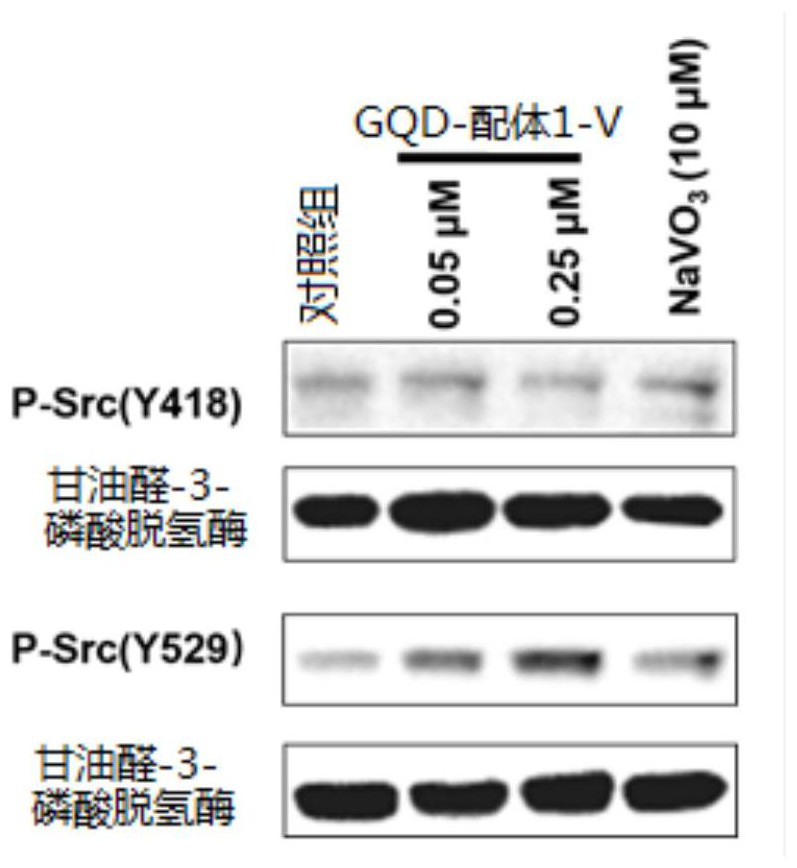
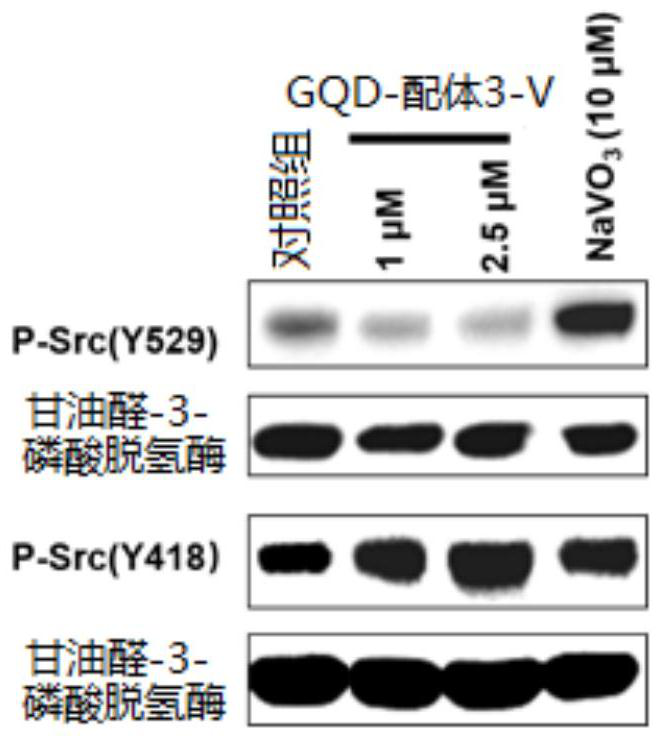
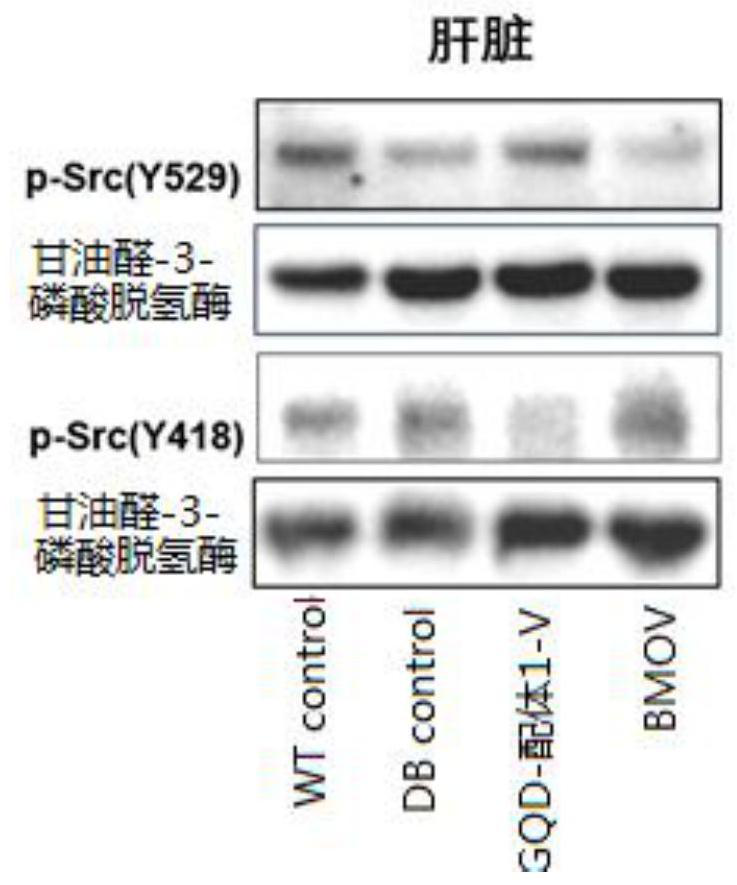
[0047] Take equal volumes of 0.012M Ligand 1 and 0.024M NaVO 3 After mixing, the ligand 1-V complex was obtained, and then the GQDs aqueous solution was stirred overnight at a ratio of 0.6 mg of ligand 1 to 1 mg of GQDs, and the reaction solution was passed through a 0.02 μm filter membrane to obtain a GQD-ligand 1-V solution. Collect the prepared GQD-ligand 1-V solution, and finally use Sephadex TMG-25M (GE Healthcare, USA) to remove unbound ligands 1 and V.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com