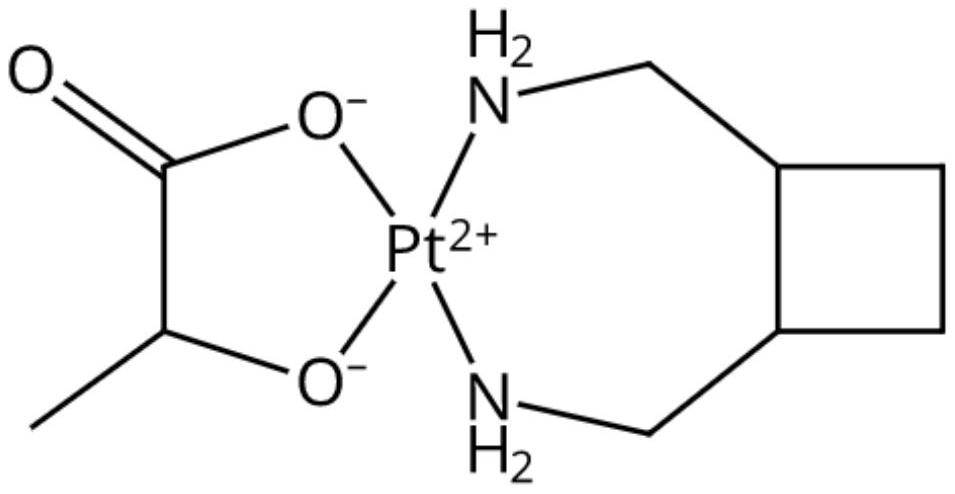
Preparation method of trans-1, 2-diaminomethyl cyclobutane and hydrochloride thereof
A technology of diaminomethylcyclobutane and hydrochloride is applied in the field of preparation of lobaplatin intermediates, and can solve the problems of poor stability, poor operability and safety, affecting the quality and yield of lobaplatin products and the like, and achieves the The effect of excellent quality, avoidance of high-risk operations, and suitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] 1. Compound I (trans-N 1 ,N 2 -dibenzylcyclobutane-1,2-dicarboxamide) preparation
[0108]Add 30.0g trans-1,2-cyclobutanedioic acid (208mmol) and 174.1g HATU (458mmol) into a 2.0L reactor, add 450ml DMF, stir to dissolve and disperse, cool down to -10~10°C, stir rapidly, drop slowly Add DIPEA80.7g (624mmol), temperature control ≤ 25 ℃, nitrogen protection, dropwise, activation reaction 0 ~ 3h. After the activation is completed, after cooling down to -10-10°C, add 49.07g of benzylamine (458mmol), and control the temperature not to exceed 40°C. After dropping, when a large amount of white solids were precipitated, the stirring reaction was continued for 2 h. Turn off the stirring, pour the reaction liquid into 72L of purified water to quench, control the temperature not to exceed 30°C, complete the addition, stir, filter and drain, collect the filter cake and beat it with purified water, filter, drain, collect the filter cake, and place Dry in a blast drying oven at 4...
Embodiment 2
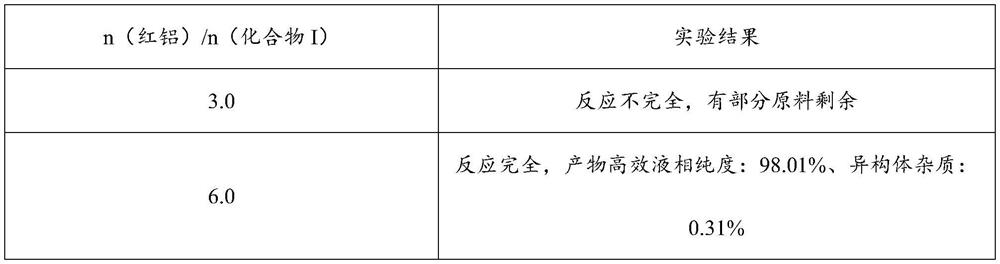
[0121] Carry out a series of experiments according to the same steps of Example 1, in the preparation process of compound II, change the addition amount of red aluminum under the situation that other conditions are constant, examine n (red aluminum) / n (compound I)=3.0,6.0 Experimental effect, and carry out follow-up experimental operation according to embodiment 1, the result is as shown in the following table:
[0122] Table 1 Experimental results of different red aluminum additions
[0123]
Embodiment 3
[0125] 1. Compound I (trans-N 1 ,N 2 -dibenzylcyclobutane-1,2-dicarboxamide) preparation
[0126] Add 600.04g trans-1,2-cyclobutanedioic acid (4.16mol) and 3482.10g HATU (9.16mol) to a 100L reactor, add 9000ml DMF, stir to dissolve and disperse, lower the temperature to -10~10℃, stir rapidly, slowly Add DIPEA1614.39g (12.49mol) dropwise, temperature control ≤ 25°C, nitrogen protection, after dropping, activate the reaction for 0-3h. After the activation is completed, the temperature is lowered to -10 to 10°C, and 981.48 g of benzylamine (9.16 mol) is added to control the temperature not to exceed 40°C. After dropping, when a large amount of white solids were precipitated, the stirring reaction was continued for 2 h. Turn off the stirring, pour the reaction liquid into 72L of purified water to quench, control the temperature not to exceed 30°C, complete the addition, stir, filter and drain, collect the filter cake and beat it with purified water, filter, drain, collect the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com