Application of higenamine hydrochloride in preparation of medicine for treating osteoporosis
A technology for higenamine hydrochloride and osteoporosis, which is applied in the field of chemical drug development, can solve the problems of different diseased organs and different pathogenic mechanisms, and achieves the effect of promoting osteogenesis, less side effects, and promoting osteogenic differentiation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
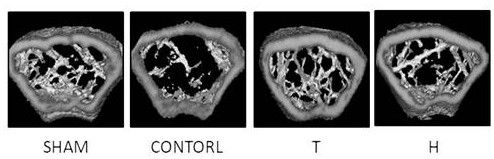
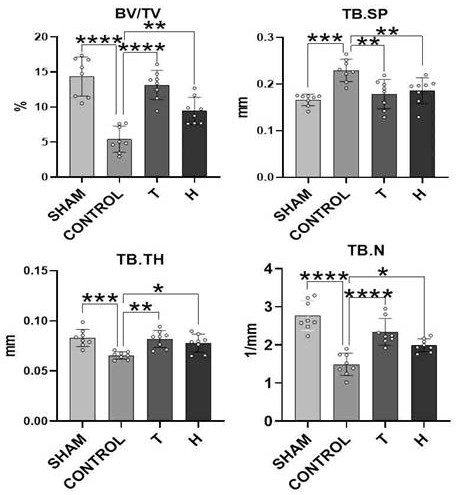
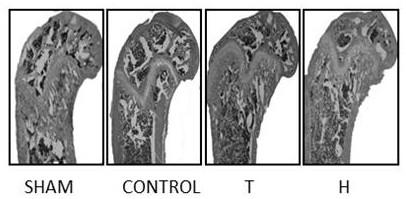
[0047] Embodiment 1: In vivo functional experiment of higenamine hydrochloride
[0048]Experimental method: 12-week-old C57BL / 6J female mice were randomly divided into four groups, namely ovariectomized intragastric administration of PBS solution group (CONTROL group), ovariectomized subcutaneous injection of teriparatide group (T group, the drug solvent was PBS solution), ovariectomized higagenamine hydrochloride group (group H, drug solvent is PBS solution), sham operation group (SHAM group), 10 rats in each group. Inject 3% pentobarbital sodium (35mg / kg) intraperitoneally. After anesthesia, fix on a special animal experiment table in prone position, cut off the local body hair in the operation area, sterilize with iodine, spread sterile towels, and take the back of the mice respectively. The lateral paramedian incision was made, and the bilateral ovaries and fallopian tubes were separated layer by layer. The mice in the CONTROL group, T group, and H group were ligated the ...
Embodiment 2
[0061] Embodiment 2: In vitro functional experiment of higenamine hydrochloride
[0062] experimental method:
[0063] (1) In vitro culture of mouse primary mesenchymal stem cells
[0064] ①Take a 6-8 week old C57 / B6 mouse, remove the mouse femur and tibia after anesthesia, and remove the muscle;
[0065] ②Take autoclaved 300μL and 1.5mL centrifuge tubes, and pierce the bottom of the small tubes;
[0066] ③ Cut off the two ends of the separated lower limb bone, put it in a small tube, add 100 μL of complete medium, and put the small tube in the big tube;
[0067] ④ Centrifuge at 12000rpm, resuspend the cells centrifuged into the large tube, centrifuge at 1000rpm, discard the supernatant, resuspend the cells and seed the plate;
[0068] ⑤After the cell confluence reaches 70%-80%, start the intervention treatment.
[0069] (2) Interfering with the differentiation of mesenchymal stem cells into osteoblasts
[0070] After the cell confluence was 70%-80%, the medium was replac...
Embodiment 3
[0081] Example 3: In vitro pathway verification of higenamine hydrochloride
[0082] Experimental method: Take mouse osteoblast precursor cells MC3T3-E1 in the logarithmic growth phase, inoculate them into 6-well plates, and place them at 37°C and 5% CO 2 In the incubator with saturated humidity, when the culture medium (a-MEM, 10% FBS, 1% double antibody) was used to culture the cells to grow to 70%-80%, the CONTROL group was given DMSO, and the T group was given TGF-β protein (drug The solvent was DMSO, with a final concentration of 10 ng / mL), and group H was given higenamine hydrochloride (the drug solvent was DMSO, and the final concentrations of samples used for Western Blot detection were 0.08, 0.4, 2, 10, 50, and 250 μM, respectively, with The final concentration of the sample detected by immunofluorescence was 10 μM), placed in the incubator for 6 hours, and the relevant indicators of the TGF-β pathway were detected by Western Blot and immunofluorescence.
[0083] Det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com