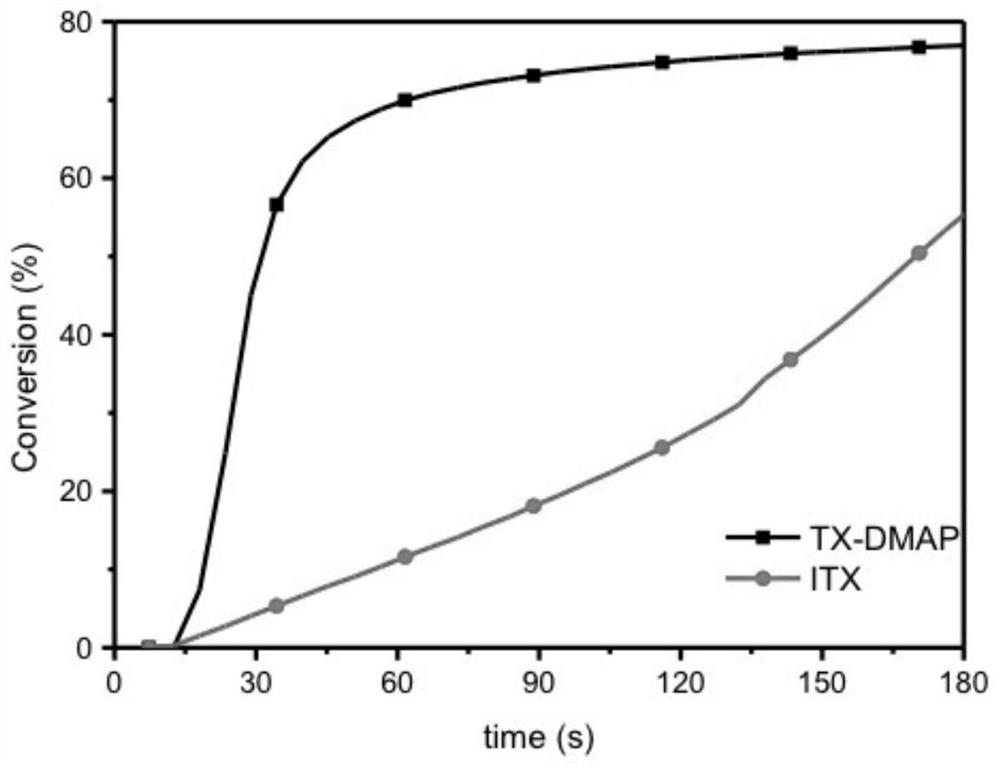
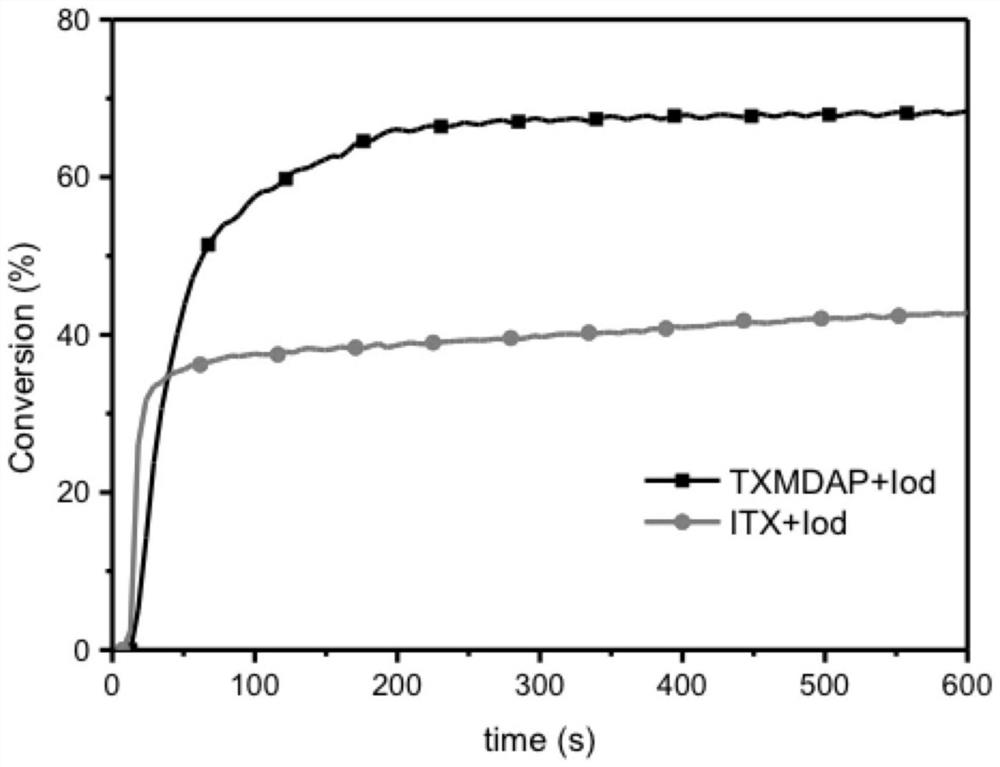
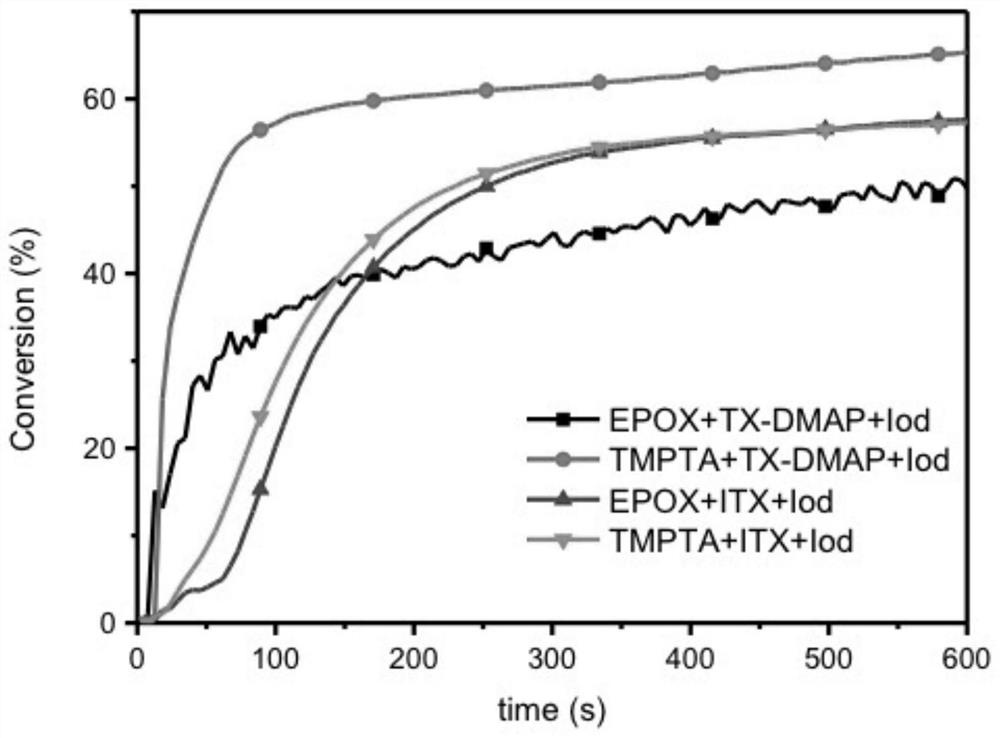
Thioxanthone-chalcone photoinitiator as well as preparation method and application thereof
A technology of photoinitiator and thioxanthone, applied in the direction of organic chemistry, can solve the problems of small volume shrinkage, cationic polymerization inhibition, long dark reaction, etc., and achieve the effect of simple synthesis method, high initiation activity and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 2-(3-(4-(dimethylamino)phenyl)-3-carbonyl-1-en-1-yl)-thioxanthone (TX-DMAP)
[0027] 2-formyl thioxanthone (2.40g, 10.0mmol), 4-dimethylaminoacetophenone (1.79g, 11mmol) were charged in a 250ml three-necked flask equipped with a reflux condenser, a thermometer, and a stirring bar, Sodium hydroxide (the amount of sodium hydroxide used is such that the concentration of alkali in the reaction system is 16% (m / m)), 50% v / v ethanol 36mL, stirred at room temperature, and TLC detected that the reaction was complete after 3.0h. Filter, wash the filter cake with water and ethanol, and dry to obtain 3.35 g of pure product with a yield of 87%.
[0028] The pure product verified by infrared data and proton nuclear magnetic resonance spectrum data is 2-(3-(4-(dimethylamino)phenyl)-3-carbonyl-1-en-1-yl)-thioxanthone. Among them, the proton nuclear magnetic resonance spectrum data is HNMR (CDCl 3 ,400MHz): 3.13(s,6H,NCH 3 ), 6.72-6.78(d, 2H, J=9.2Hz), 7.52-7.58(t, 1H...
Embodiment 2
[0030] Preparation of 2-(3-(4-(dimethylamino)phenyl)-3-carbonyl-1-en-1-yl)-thioxanthone (TX-DMAP)
[0031]2-formyl thioxanthone (2.40g, 10.0mmol), 4-dimethylaminoacetophenone (1.96g, 12mmol) were charged in a 250ml three-necked flask equipped with a reflux condenser, a thermometer, and a stirring bar, Potassium carbonate (the amount of potassium carbonate used is such that the concentration of alkali in the reaction system is 16% (m / m)), 30 mL of ethanol, stirred at room temperature, and the reaction was detected by TLC after 12 hours. Filter, wash the filter cake with water, wash with ethanol, and dry to obtain 3.27 g of pure product with a yield of 85%. The obtained pure product was confirmed to be TX-DMAP by IR and H-NMR data.
Embodiment 3
[0033] Preparation of 2-(3-(4-(dimethylamino)phenyl)-3-carbonyl-1-en-1-yl)-thioxanthone (TX-DMAP)
[0034] 2-formyl thioxanthone (2.40g, 10.0mmol), 4-dimethylaminoacetophenone (2.12g, 10.5mmol) were charged into a 250ml three-necked flask equipped with a reflux condenser, a thermometer, and a stirring bar. , potassium bicarbonate (the amount of potassium bicarbonate used is such that the concentration of alkali in the reaction system is 16% (m / m)), 45 mL of N,N-dimethylformamide, stirred at room temperature, and TLC detected that the reaction was complete after 10 h. Filter, wash the filter cake with water, wash with ethanol, and dry to obtain 3.0 g of pure product with a yield of 78%. The obtained pure product was confirmed to be TX-DMAP by IR and H-NMR data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com