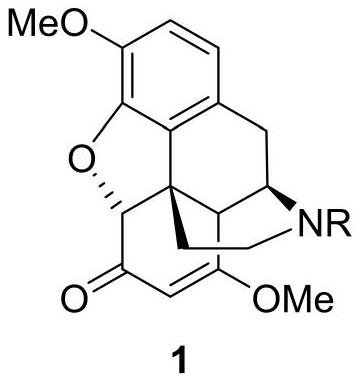
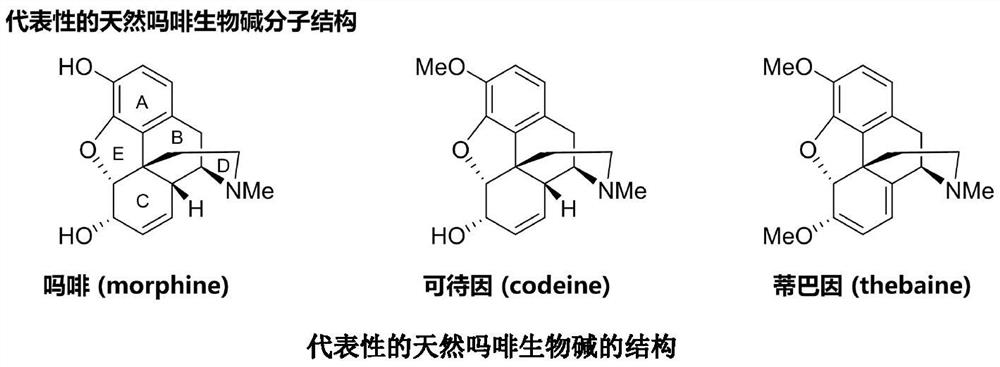
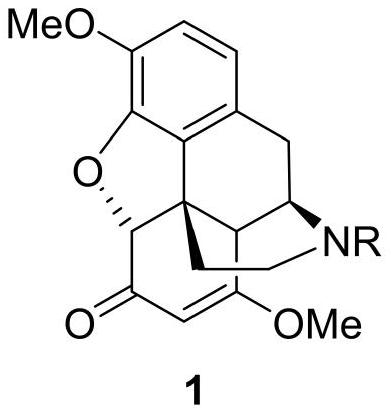
Thebaine derivative as well as preparation method and application thereof
A derivative, thebaine technology, applied in the field of drug synthesis, can solve the problems of no practical significance and production value, and achieve the effect of simple operation, strong operability and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] The preparation of embodiment 1 compound 10, reaction scheme is as follows:
[0089]
[0090] Include the following steps:
[0091] Compound 9 (20.0g, 40.4mmol) was dissolved in 200mL of glacial acetic acid and reacted at 90°C for 40h. LC / MS detection showed that the raw materials disappeared completely, and the reaction solution was cooled to room temperature and stirred for 30 min. The reaction solution was filtered to obtain a gray solid. The obtained filtrate was concentrated and recrystallized from ethyl acetate to obtain off-white solid. The solids obtained above were combined, dispersed in 10 mL of ethyl acetate, stirred at room temperature for 5 min, filtered and dried in vacuo to obtain white solid compound 10 (13.6 g, yield 85%), which was directly used in the next reaction without purification.
[0092] Compound 10: 1 H NMR (400MHz, CDCl 3 )δ7.70(d, J=8.0Hz, 2H), 7.43(s, 1H), 7.25(d, J=8.0Hz, 2H), 6.74(d, J=8.4Hz, 1H), 6.59(d, J=8.4Hz, 1H), 6.20(s, 1...
Embodiment 2
[0093] Example 2 Synthesis of thebaine derivative 1, the reaction scheme is as follows:
[0094]
[0095] Include the following steps:
[0096] Compound 10 (2.50g, 5.03mmol, 1.0equiv) was dissolved in 40mL of methanol / dichloromethane (1:1), and sodium borohydride (382mg, 10.06mmol, 2.0equiv) was slowly added at 0°C, and the addition was completed. The reaction solution was gradually raised to room temperature for 30 min. After TLC showed that the reaction was complete, a saturated ammonium chloride solution (30 mL) was added to the reaction solution under an ice bath to quench the reaction, extracted with dichloromethane (30 mL×3), the organic layers were combined, dried over anhydrous sodium sulfate, filtered, and concentrated The crude compound 11 (2.65 g) was obtained as a white foam.
[0097] Under the protection of argon, the above crude compound 11 was dissolved in 15 mL of dry 1,4-dioxane, and N,N-dimethylformamide dimethyl acetal (15 mL, 0.11 mol) was added to rea...
Embodiment 3
[0100] The synthesis of embodiment 3 compound 5, reaction scheme is as follows:
[0101]
[0102]Thebaine derivative 1 (1.7g, 3.64mmol, 1.0equiv) was dispersed in dry ethylene glycol dimethyl ether (40mL) under the protection of argon, and lithium aluminum hydride (1.0M / LinTHF, 18.2 mL, 5.0 equiv.), after the addition, the reaction solution was gradually raised to room temperature for 40 hours. TLC detection showed that the raw material disappeared completely, and 5 mL of isopropanol was added to the reaction solution under an ice bath, and 0.7 mL of water was slowly added after stirring for 5 min, and 0.7 mL of 15% aqueous sodium hydroxide solution was added after stirring for 5 min, and 2 mL of water was added after stirring for 5 min. The reaction solution was raised to room temperature and stirred for 30 min. The reaction solution was filtered, the filter cake was washed 6 times with dichloromethane, and the filtrate was concentrated to obtain an off-white powder (crud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com