Synthesis method of bivalirudin
A technology of bivalirudin and a synthesis method, applied in the field of polypeptide drug preparation, can solve the problems of low expression efficiency, application limitation, difficulty in product extraction and recovery, etc., and achieve the effects of improving utilization rate, increasing yield, and reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
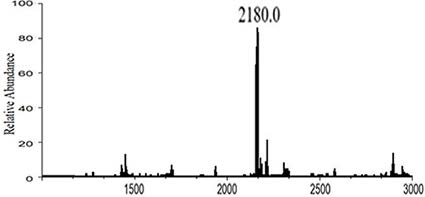
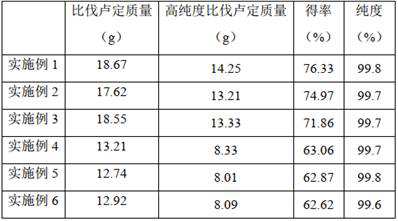
Image
Examples
Embodiment 1
[0046] Synthesis of Bivalirudin
[0047] 1. Activation of resin
[0048] Take 45g of Wang resin and add 690g of activation solution, 100r / min oscillating reaction for 2h; after the reaction, filter out the resin, wash and dry to obtain the activated resin; the activation solution includes 600g of methylene chloride, 60g of B-butyrolactone, Nadic anhydride 30g.
[0049] 2. Synthesis of Leu-resin
[0050] Take 50g of swelled activated resin and 0.08mol Fmoc-Leu, add 0.08mol DMF to swell, then ice-bath, continue to add 0.08mol DCC, 0.08mol HOBt and 0.0025mol DMAP, after 2h in ice-bath, 4°C, 100r / min stirring overnight; After stirring, filter, wash and dry to obtain Fmoc-Leu-resin; add 100g of pyridine / dichloromethane / DMF mixed solution (V / V / V=1:1:3) to Fmoc-Leu-resin, react for 30min, suction filter and Wash and dry to obtain Leu-resin.
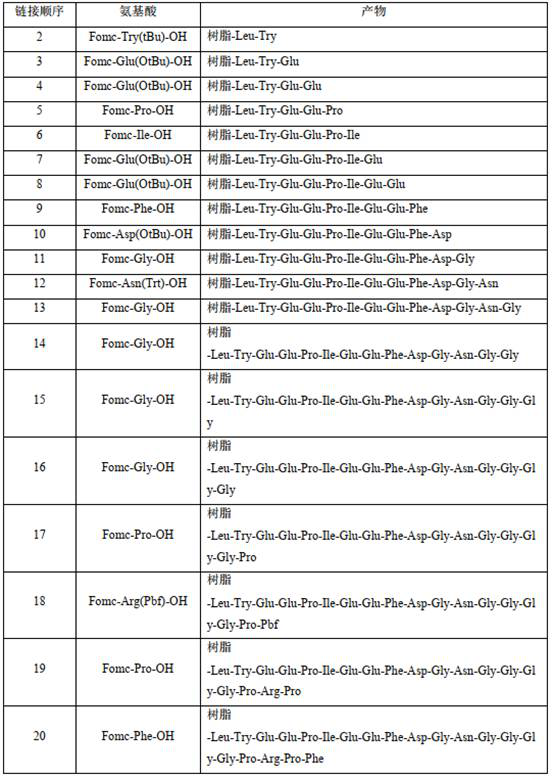
[0051] 3. Synthesis of bivalirudin-resin
[0052] According to the operation method in step 2, amino acids were sequentially coupled to pr...
Embodiment 2
[0062] Synthesis of Bivalirudin
[0063] 1. Activation of resin
[0064] Take 30g of Wang resin and add 573g of activation solution, oscillate at 100r / min for 1h; filter out the resin after the reaction, wash and dry to obtain the activated resin; the activation solution is 540g of dichloromethane, 30g of B-butyrolactone, Nadic anhydride 3g.
[0065] 2. Synthesis of Leu-resin
[0066] Take 30g of swelled activated resin and 0.05mol Fmoc-Leu, add 0.05mol DMF to swell, then ice-bath, continue to add 0.05mol DCC, 0.05mol HOBt and 0.001mol DMAP, after 1h in ice-bath, stir overnight at 2°C at 100r / min; After stirring, filter, wash and dry to obtain Fmoc-Leu-resin; add 90g of pyridine / dichloromethane / DMF mixed solution (V / V / V=0.25:1:1) to Fmoc-Leu-resin, react for 20min, suction filter and Wash and dry to obtain Leu-resin.
[0067] 3. Synthesis of bivalirudin-resin
[0068] With embodiment 1.
[0069] 4. Deprotection
[0070] Take 100g of 90% TFA solution, 10g of (S)-glycidol...
Embodiment 3
[0074] Synthesis of Bivalirudin
[0075] 1. Activation of resin
[0076] Take 60g of Wang resin and add 915g of activation solution, oscillate at 120r / min for 4h; filter out the resin after the reaction, wash and dry to obtain the activated resin; the activation solution is 780g of methylene chloride, 90g of B-butyrolactone, Nadic anhydride 45g.
[0077] 2. Synthesis of Leu-resin
[0078] Take 60g of swollen activated resin and 0.15mol Fmoc-Leu, add 0.15mol DMF to swell, then ice-bath, continue to add 0.15mol DCC, 0.15mol HOBt and 0.005mol DMAP, after 5h in ice-bath, stir overnight at 4°C at 120r / min; After stirring, filter, wash and dry to obtain Fmoc-Leu-resin; add 120g of dichloromethane / DMF mixed solution (V / V / V=1:1:3) to Fmoc-Leu-resin and react for 50min, then suction filter and wash and dry Dry to obtain Leu-resin. (modified according to the content of the invention)
[0079] 3. Synthesis of bivalirudin-resin
[0080] With embodiment 1.
[0081] 4. Deprotection ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com