Preparation method of mirabegron key intermediate
A key and integrated technology, which is applied in the field of preparation of key intermediates of Mirabegron, can solve problems such as production safety risks not being effectively resolved, affecting the quality of Mirabegron finished products, and strong dependence on equipment, etc., to achieve easy scale The effects of high performance amplification, less impurities, high product purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
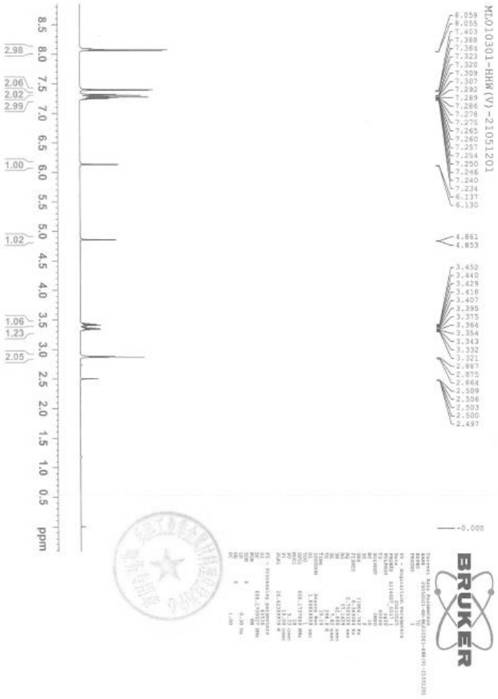
[0038] Embodiment 1: preparation of formula (V) compound
[0039]
[0040] Add 200 g of compound of formula (VII) and 286 g of compound of formula (VI) into 1.6 kg of N,N-dimethylformamide, and add 132 g of triethylamine, 178 g of 1-hydroxybenzotriazole and 264 g of EDCI at room temperature. Stir the reaction at room temperature for 1 h, add 1.6 kg of purified water, stir and crystallize for 2 h, filter, and dry to obtain 371 g of the compound of formula (V), with a yield of 93.7%.
Embodiment 2
[0041] Embodiment 2: preparation of formula (IV) compound
[0042]
[0043] Add 300 g of the compound of formula (V) into 1.5 kg of tetrahydrofuran, and slowly add 675 g of 2 mol / L borane dimethyl sulfide complex. After the addition, the temperature was raised to reflux for 3 hours, and the reaction ended. Cool down to room temperature and add 120 g of methanol and 200 g of hydrochloric acid. The temperature was raised to 60°C and stirred for 1 hour. The solvent was evaporated under reduced pressure, 1.2 kg of methanol was added after concentration, stirred for 2 hours, suction filtered, and air-dried at 50±5°C to obtain 300 g of the compound of formula (IV), with a yield of 93.2%.
Embodiment 3
[0044] Embodiment 3: preparation of formula (III) compound
[0045]
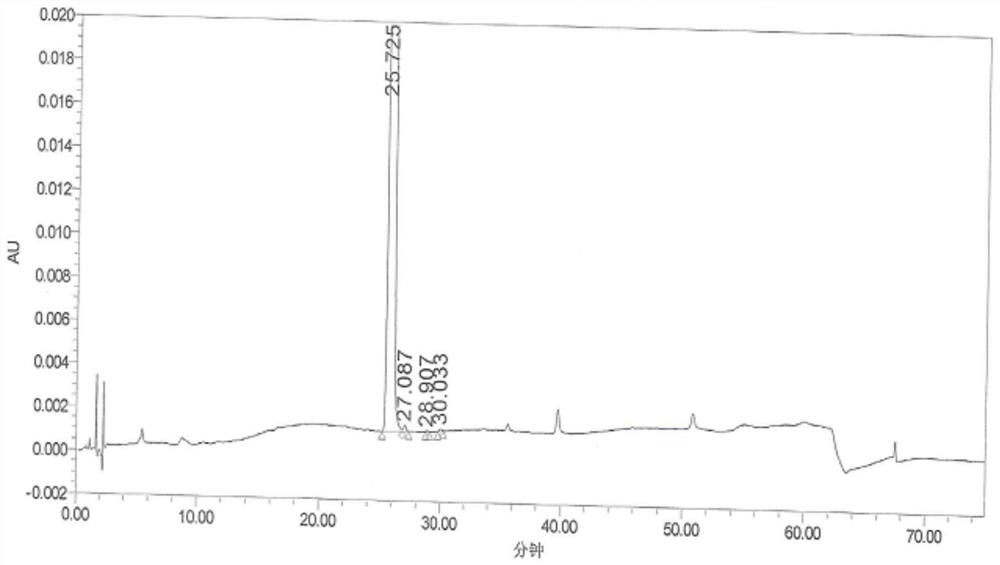
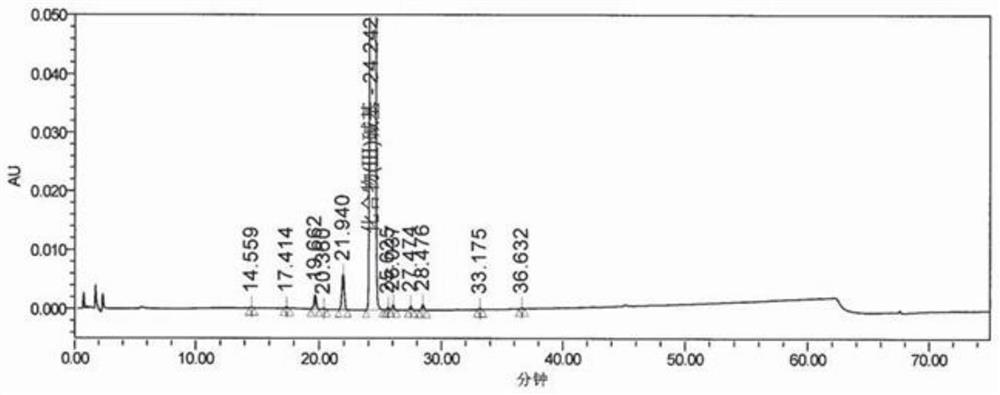
[0046] Add 200g of the compound of formula (IV) into 1600g of methanol, add 16.8g of ferric chloride hexahydrate, and 60.0g of activated carbon; keep stirring for 30 minutes, slowly add 234g of hydrazine hydrate, dropwise, heat up to reflux and keep warm for 5h, and the reaction is complete , lower the temperature to 25±5°C, and obtain the filtrate by suction filtration; add the filtrate to the reaction flask, slowly add 3.0kg of purified water, and gradually solids precipitate; keep warm at 10±5°C for crystallization for 2 hours, suction filter, and dry to obtain formula (III) Compound 151g; Yield 94.9%. Such as figure 1 , shown in table 1, impurity formula (VIII) compound, impurity formula (IX) compound and impurity formula (X) compound all do not detect.
[0047] The formula (III) compound chromatographic peak result that table 1 embodiment 3 prepares
[0048] keep time area %area ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com