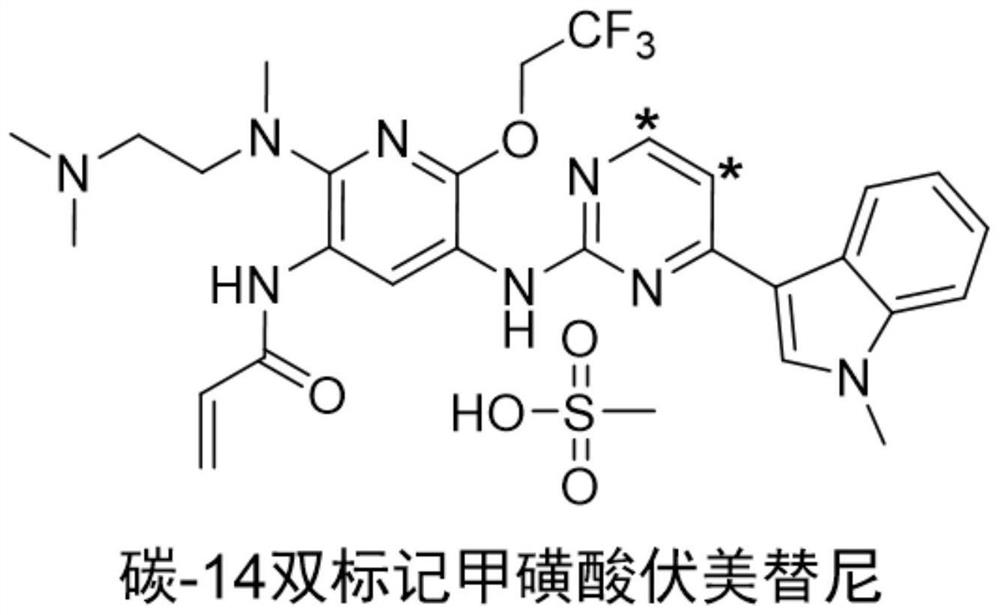
Synthesis method of radioisotope carbon-14 double-labeled furmonertinib mesylate
A technology of radioisotope and synthesis method, applied in the field of radiochemical synthesis, can solve problems such as difficulty in reaching detection limit, inability to increase the dosage of fumetinib mesylate, failure of tracer test and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
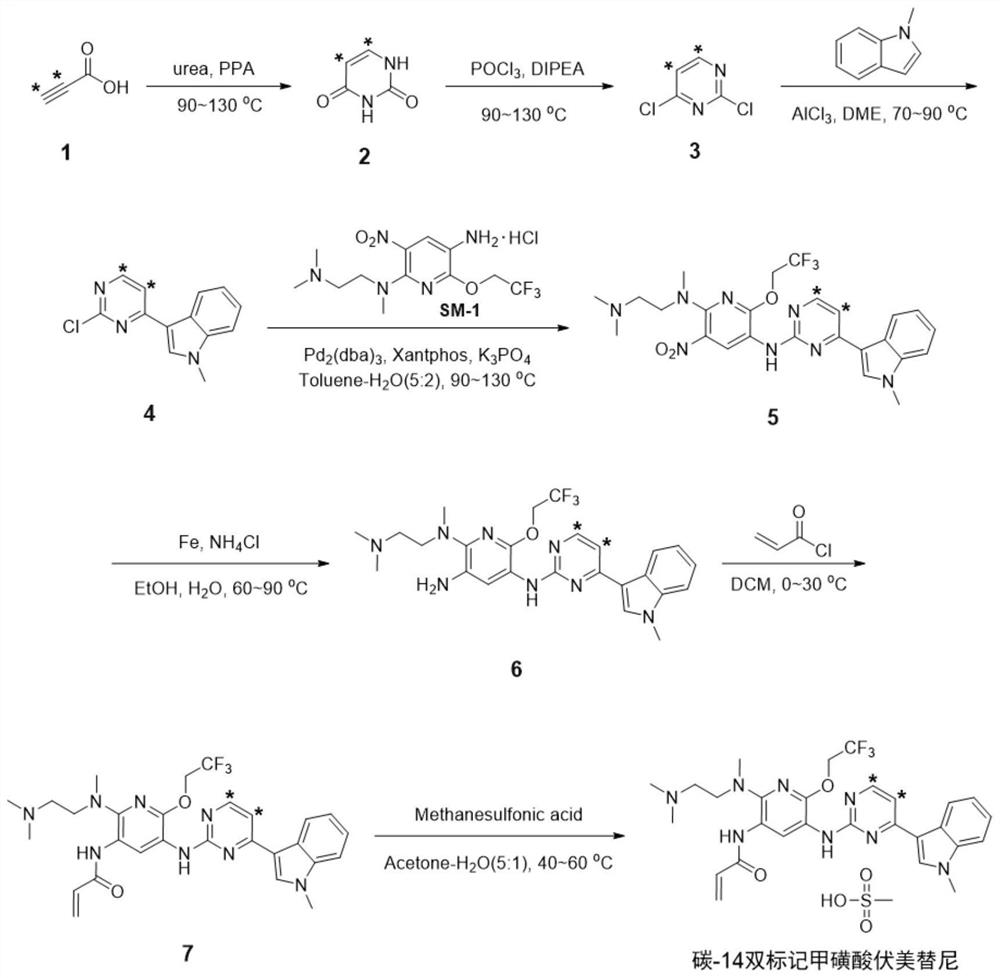
[0036] refer to Figure 2 to Figure 9 , specifically, the synthesis method of the radioactive isotope carbon-14 double-labeled vormetinib mesylate comprises the following steps:
[0037] S1: Under inert gas protection and 90-130°C, [2,3- 14 C 2 ] propiolic acid (1) React with urea in polyphosphoric acid (PPA).
[0038] After the reaction, dilute with water, adjust pH with alkali and filter to obtain [5,6- 14 C 2 ] uracil (2) ;
[0039] S2: Under inert gas protection and 90-130°C, the intermediate [5,6- 14 C 2 ] uracil (2) React with phosphorus oxychloride under the catalysis of diisopropylethylamine (DIPEA). After the reaction, the reaction mixture was purified by conventional post-processing and column chromatography to obtain 2,4-dichloro[5,6- 14 C 2 ] pyrimidine (3) ;
[0040] S3: Under inert gas protection and 70-90 °C, the intermediate 2,4-dichloro[5,6- 14 C 2 ] pyrimidine (3) , N-methylindole and Lewis acid in an aprotic organic solvent. After the react...
Embodiment 1
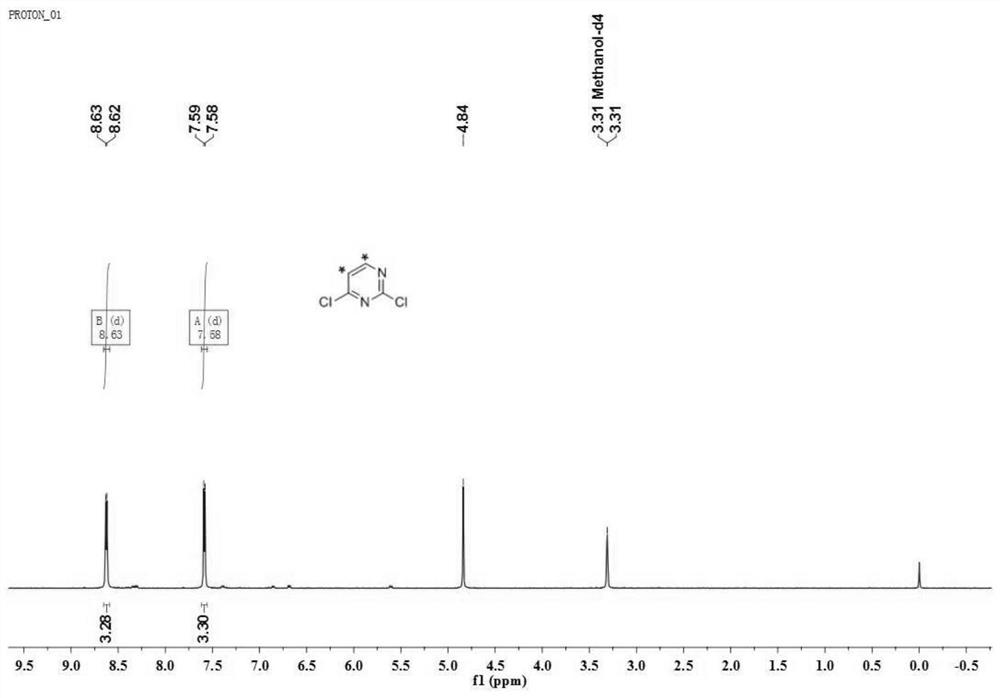
[0052] Under argon protection and 50°C, urea (50 mg) and polyphosphoric acid (1.5 mL) were stirred and dissolved, and [2,3- 14 C 2 ] propiolic acid (1,80.4 μCi, 750 μmol). After the addition, the temperature was raised to 90°C, and the reaction was completed after stirring for 16 hours. 1 mL of water was added to the reaction liquid to quench the reaction, the pH was adjusted to about 7 with ammonia water (28-30% wt), and the crystallization was carried out by stirring at 5° C. for 4 h. Filtration, the obtained solid was rinsed with 1mL water and 5mL methanol successively, and after drying, the solid [5,6- 14 C 2 ] Uracil (2, 65.2 μCi, 81%). 1 H NMR (400MHz, DMSO-d 6 )δ10.87(s,2H),7.38(d,J=7.6Hz,1H),5.45(d,J=7.6Hz,1H).ESI-MS(m / z):135,139[M+H] + .
[0053] Under the protection of argon, the [5,6- 14 C 2 ]Uracil (2,63.2μCi) and 2 drops of diisopropylethylamine were added in turn to phosphorus oxychloride (3mL), heated to 90°C and stirred for 2.5h. The reaction solution...
Embodiment 2
[0060] Under argon protection and 50°C, urea (50 mg) and polyphosphoric acid (1.5 mL) were stirred and dissolved, and [2,3- 14 C 2 ] propiolic acid (1,80.4 μCi, 750 μmol). After the addition, the temperature was raised to 110°C, and the reaction was completed after stirring for 13 hours. Add 1 mL of water to the reaction liquid to quench, add dropwise a saturated aqueous solution of sodium bicarbonate to adjust the pH to about 7, stir at 5°C for 4 h to crystallize, filter, and rinse the solid with 1 mL of water and 5 mL of methanol in sequence. Dried to a solid [5,6- 14 C 2 ] Uracil (2, 64.2 μCi, 80%). ESI-MS(m / z):135,139[M+H] + .
[0061] Under the protection of argon, the [5,6- 14 C 2 ]Uracil (2,63.2μCi) and 2 drops of diisopropylethylamine were added in turn to phosphorus oxychloride (3mL), heated to 110°C, and stirred for 1.5h. The reaction solution was cooled to room temperature, and after conventional treatment (same as in Example 1), 2,4-dichloro[5,6- 14 C 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com