Vesicle for treating diseases caused by helicobacter pylori
A technology of Helicobacter pylori and vesicles, applied in the field of medicine, can solve problems such as infection recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 vesicle
[0027] Thin-film dispersion method: Weigh water-soluble or fat-soluble RHL, Chol, CLR and calcitriol, respectively, and dissolve them in chloroform for ultrasonic dispersion. Mix the dispersed RHL solution (containing 1 mg RHL), Chol solution (containing 0.5 mg Chol), CLR solution (containing 8 μg CLR) and calcitriol solution (containing 33.3 μg calcitriol) and add to the round bottom flask , after vacuum rotary evaporation, hydration and ultrasonic dispersion, and pass through a 0.22 μm filter membrane.
Embodiment 2
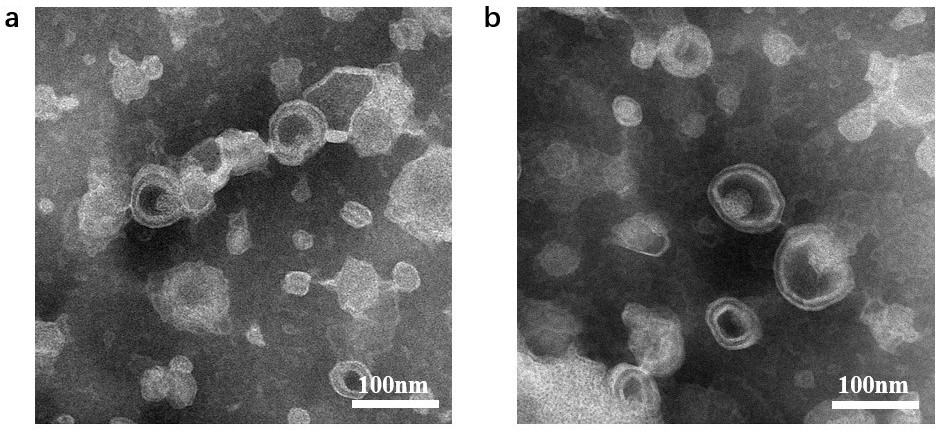
[0028] Example 2 Characterization of multifunctional vesicles
[0029] Encapsulation efficiency: The drug-loaded vesicles prepared in Example 1 were injected into the vesicles after methanol demulsification. Chromatographic conditions: chromatographic column is Agilent ZORBAX SB-C18 (250×4.6 mm, 5 μm); acetonitrile is used as mobile phase A, phosphate buffer is used as mobile phase B, and elution is carried out according to A:B= 48:52; flow rate The volume is 1 ml per minute; the column temperature is 45°C; the detection wavelength is 210 nm. The encapsulation efficiency was calculated according to the following formula.
[0030] Encapsulation rate (%) =
[0031] in, W1 is the drug content encapsulated in the vesicles, W0 is the amount of drug added to the system.
[0032]Experimental results: the drug encapsulation efficiency of vesicles made of water soluble RHL (water soluble-RHL vesicle, WRV) and liposoluble RHL vesicles (liposoluble-RHL vesicle, LRV) are shown in T...
Embodiment 3
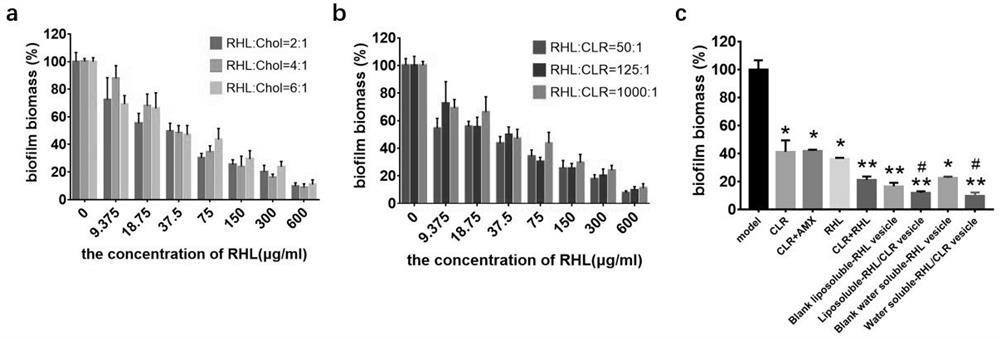
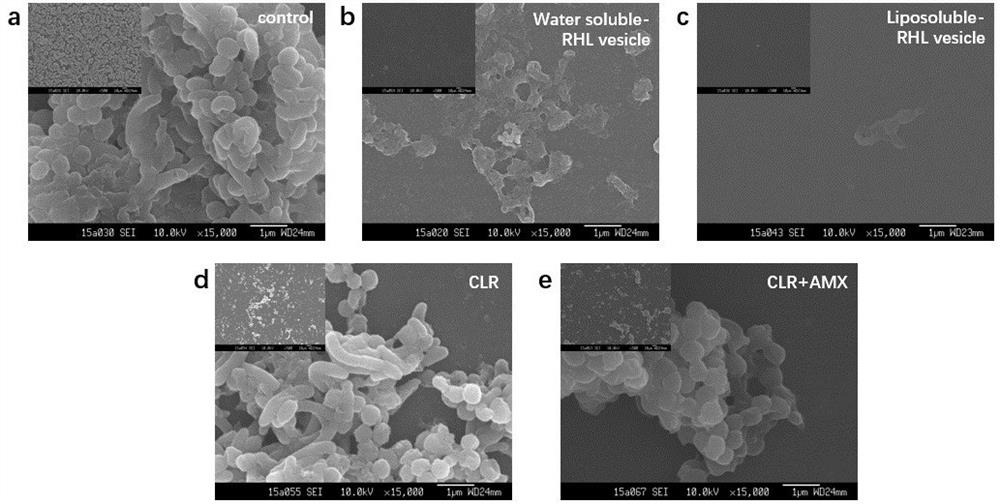
[0038] Example 3 The scavenging effect of vesicles on bacterial film
[0039] (1) Dilute with Brain Heart Infusion Broth (BHI) containing 2% FBS H. pylori bacterial suspension to OD 600nm The value is 0.2, and inoculated in a 48-well plate, and put into a microaerophilic gas bag of the corresponding specification to maintain a suitable gas environment (5% O 2 , 85% N 2 , 10%CO 2 ), and cultured in a 37°C incubator for 72 h. (2) Drug treatment: ① In order to explore the influence of the main components in the vesicles on the removal of bacterial film, the present invention prepared vesicles with different contents of Chol and CLR while keeping the content of RHL unchanged. The drug-loaded vesicles were diluted so that the highest concentration of vesicles in each group was consistent, all containing 600 μg / ml RHL, and diluted according to the double method. Take the cultured pellicle out of the airtight jar and wash it with sterile PBS to remove free H. pylori Afterward...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com