Optimized culture medium, kit and culture method for human placenta-derived mesenchymal stem cells
A technology for optimizing medium and stromal cells, applied in the field of optimizing medium for human placenta-derived mesenchymal stem cells, can solve the problems of different culture systems, inability to obtain MSCs, etc., achieve significant exponential growth, reduce large-scale cell preparation and Production cost, effect of large colony formation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
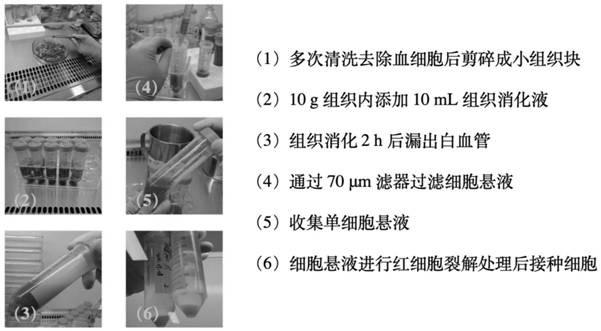
[0065] 1. Collection of placental tissue
[0066] (1) After the fetus is delivered, the placenta is completely separated from the mother, and the placenta is fully washed with normal saline, and then the surface is quickly rinsed with 75% medical alcohol.
[0067] (2) Quickly put the placenta into a tissue preservation bag, add tissue preservation solution (DMEM containing 4,500 mg / mL glucose and antibiotic solution (0.1% gentamicin, 0.2% streptomycin, and 0.12% penicillin)) to fully infiltrate , into the placenta collection box.
[0068] (3) Store the tissue preservation bag in a tissue transport box at 4°C and transport it to the laboratory. Samples were processed within 2 to 4 hours after tissue collection, and all operations were performed in a sterile laboratory environment.
[0069] (4) Place the sample in a medical stainless steel tray in a biological safety cabinet. Using a needle and syringe, rinse the tissue surface several times with 4°C pre-cooled D-PBS to remov...
Embodiment 2
[0110] (1) The placental mesenchymal stem cell culture method of the present invention has the stability of repeated application
[0111] The present invention sets up three isolation and expansion groups of placental tissue mesenchymal stem cells from different tissue donor sources, conducts microscopic examination of cell morphology and calculation of the total amount of preparation in the middle and later stages of the experiment, and verifies whether the cultivation method of the present invention can be realized Stable isolation and expansion of placenta-derived mesenchymal stem cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com